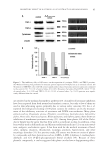
INHIBITORY EFFECTS OF N-FERULOYLDOPAMINE 137 Forward primer Reverse primer Actin GTGGGGCGCCCCAGGCACCA CTCCTTAATGTCACGCACGATTTC Pmel17 gene GGTGGAGACCACAGCTAGAGA GCGGAACCTGCCCAAGGCCTGCT One-step quantitative real-time reverse transcriptase polymerase chain reaction (qRT- PCR) was performed using the “iScript One-Step RT-PCR Kit with SYBR Green” (Biorad, Marnes-la-Coquette, France). The reaction mix contained the SYBR Green buffer 1× the two specifi c primers at 0.6 μM 1 μl of enzyme mix 50 ng of total sample RNA and qsp 50 μl with RNase-free water. The reaction was run in 96-well plates using CHROMO4® thermocycler (Biorad). Results were normalized to the actin transcript levels. Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Dunnett’s procedure for multiple comparisons versus untreated control group. RESULTS AND STATISTICAL ANALYSIS Results are presented as means ± SD for experiments conducted at least in triplicate (n = 3). The statistical signifi cance between groups was assessed using Student’s t-test for positive controls and one-way ANOVA followed by either Tukey’ test or Dunnett’s method for N-feruloyldopamine. RESULTS SCREENING RESULTS OF COMPOUNDS HAVING STRUCTURAL HOMOLOGY WITH TYROSINASE SUBSTRATE The conserved catalytic center of tyrosinase is composed of two copper atoms bound to six histidine residues. Tyrosinase substrates, i.e., the amino acids L -Tyrosine and L -DOPA dock to this dinuclear copper center by their phenol function and catechol group, respec- tively (16,17). Using docking approach, Khatib et al. (18) have shown that addition of a short two– carbon lipophilic alkyl chain enhanced the tyrosinase-inhibiting properties of resorcinol. In addition, we have previously shown that amides derived from p-coumaric acid with a two–carbon alkyl chain separating the amide function from a phenol ring was a potent structure for tyrosinase inhibition (19). In our approach of searching for a substrate-mimicking tyrosinase inhibitor, N- feruloyldopamine was selected as the best candidate (Figure 1). Indeed, this molecule exhibits all the structural features that are reported to support a tyrosinase–substrate in- teraction or a tyrosinase-inhibiting effect, i.e., a catechol moiety and a phenol substituent in para position of a two-carbon alkyl chain (20). EVALUATION OF N-FERULOYLDOPAMINE CYTOTOXICITY Before an evaluation of its inhibiting properties, the cytotoxicity of N-feruloyldopamine at increasing concentrations was measured both in human melanocytes and in murine
JOURNAL OF COSMETIC SCIENCE 138 B16-F10 cells. After 72 h of incubation, N-feruloyldopamine did not signifi cantly alter cell growth for concentrations up to 50 μM for B16-F10 cells and for concentrations at least up to 100 μM for NHEMs (Figure 2). HUMAN AND MUSHROOM TYROSINASE INHIBITIONS The effect of N-feruloyldopamine on tyrosinase activity was fi rst assessed in NHEM using MBTH assay (21). Results show that N-feruloyldopamine dose dependently inhibited tyrosinase activity for concentrations ranging from 5 to 100 μM (Figure 3A). From 50 μM, the inhibitory effect of N-feruloyldopamine was signifi cantly higher than that of the positive control arbutin (p 0.01). Contrary to human tyrosinase, no signifi cant inhibition of mushroom tyrosinase could be observed (Figure 3B) although the positive control kojic acid signifi cantly inhibited mushroom tyrosinase activity, thus validating the experiment. INHIBITION OF MELANIN SYNTHESIS IN B16-F10 CELLS After 72 h of incubation with N-feruloyldopamine at 10 μM, melanin synthesis was signifi - cantly decreased by 31.6% (p 0.01) compared to untreated control. N-feruloyldopamine used at 30 μM provided a signifi cant 65.5% inhibition (p 0.01) and a 75.6% inhibi- tion when used at 50 μM (p 0.01) (Figure 4). N-FERULOYLDOPAMINE ANTIOXIDANT CAPACITY To investigate further melanogenesis inhibitory action of N-feruloyldopamine in vitro, its antioxidant capacity was evaluated using DPPH in tubo assay. N-feruloyldopamine showed a dose-dependent antioxidant capacity that reached a plateau with around 70% of radical-scavenging activity for concentrations equal or above 30 μM. From 10 μM, the radical-scavenging activity of N-feruloyldopamine was comparable to that of cysteine 40 μM (Figure 5). Figure 1. Chemical structures of N-feruloyldopamine, L -tyrosine, and L -DOPA.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)