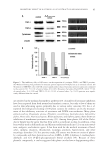
INHIBITORY MECHANISM OF RED GLOBE AMARANTH ON TYROSINASE 101 their inhibitory effect on the monophenolase activated forms of tyrosinase in vitro. Next, the best sample was selected for a dose–response study for the monophenolase and diphe- nolase activities of tyrosinase. In a 96-well plate, 70 μl of each dilution of the extract was mixed with 30 μl tyrosinase solution (333 units/ml in phosphate buffer) in triplicate. After incubation at 25°C for 5 min, 110 μl of the substrate (1.0 mM L -tyrosine or 2.0 mM L -dopa) was added to each well. The samples were incubated for 30 min at 25°C. The optical densities of the samples were then determined at 492 nm using a Sunrise plate reader (TECAN, Männedorf, Switzerland). The concentrations of the inhibitor at which half of the original tyrosinase activity was inhibited (50% inhibitory concentration, IC50) were determined for crude extract and purifi ed inhibitors. Arbutin was selected as a pos- itive control. All concentrations of the inhibitors mentioned in the study were the fi nal concentrations. The inhibition of tyrosinase activity was calculated as follows: ª « » 1 ( Inhibition 100 B Cº) A % = × A is the absorbance at 492 nm without the test sample, B the absorbance at 492 nm with the test sample, and C the absorbance at 492 nm without tyrosinase. EXTRACTION OF CRUDE EXTRACT AND PRELIMINARY SEPARATION EXPERIMENT Dried fl owers of red globe amaranth (3 kg) were extracted three times by refl ux extraction with a fi vefold, 50% (v/v) aqueous alcohol solution each incubation was performed for 2 h. After fi ltration through 0.45 μm fi lter paper, the fi ltrates were mixed and concen- trated using a rotary evaporator (Yarong Inc., Shanghai, China) at 50°C. The concen- trated extract was dispersed with distilled water until its density was approximately 1.0–1.1 g/cm3. Then, the suspended liquid was successively extracted at room tempera- ture four times each by petroleum ether, ethyl acetate, and butanol (1 liter of each solvent, 24 h), and the organic phases were concentrated to produce the extracts, which were termed PE, EA, and BA, respectively. We performed a preliminary experiment for the extracts by crudely separating them using silica gel chromatography. For PE and EA, a petroleum ether–acetic ether mixture was used as the eluent, whereas chloroform–methanol (CHCl3–MeOH) was used as the eluent for BA. The inhibitory effect of the crude sepa- rated fractions at 1 mg/ml on tyrosinase activity is listed in Table I EA was selected for further purifi cation studies. Table I Tyrosinase Inhibition by Fractions from PE, EA, and BA Tyrosinase inhibition rate (%) Fraction 1 2 3 4 5 6 7 8 9 10 PE — — — 16.58 16.38 17.93 10.35 8.70 30.06 — EA — — 30.74 44.01 72.55 92.72 33.79 53.32 33.79 41.68 BA 16.34 17.96 −24.68 −4.94 −1.53 −5.63 −2.08 −9.54 12.42 40.99
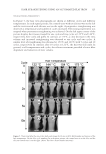
JOURNAL OF COSMETIC SCIENCE 102 PURIFICATION OF THE TYROSINASE INHIBITOR The purifi cation was based on the tyrosinase activity-guided method. The EA was sepa- rated by column chromatography over silica gel with mixtures of petroleum ether–acetic ether of increasing polarity (9:1–4:6), and 14 fractions (A–N) were collected. Fractions A–F were discarded because they were insoluble in our enzyme activity assay, and the remaining 8 fractions were separated over a Sephadex LH-20 (GE Inc., New York, NY) column with CHCl3–MeOH (1:1). The compounds with a high level of tyrosinase in- hibition were further refi ned by C18 (GE Inc.) column chromatography and eluted with methanol aqueous solutions (20%, 40%, 60%, 80%, and 100%, successively) (v/v) to prepare a white powder (approximately 30 mg), which was washed with the 40% methanol eluent. The fi nal yield of the white powder is approximately 0.01%. The overall fl ow chart of the isolation and purifi cation process is shown in Figure 1. STRUCTURE DETERMINATION The structure of the obtained compound was determined using AVANCE Digital 400 MHz NMR Spectrometer (Bruker Inc., Karlsruhe, Germany). The detailed structural information is as follows: 1 H-NMR (CDCl3,δ, ppm.) 7.56 (1H, d, J = 4 Hz), 7.54 (1H, d, J = 4 Hz), 6.83 (1H, t, J = 8 Hz), 3.90 (3H, s) 13 C-NMR (CDCl3, δ, ppm.) 170.21(s), 152.85(s), 148.85(s), 125.48(d), 123.29(s), 116.04(d), 114.08(d), and 56.63(q). Previous reports (17,18) have identifi ed this compound as VA. Figure 1. Flow chart of isolation of tyrosinase inhibitors from red globe amaranth.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)