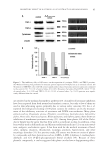
J. Cosmet. Sci., 64, 99–110 (March/April 2013) 99 Inhibitory mechanism of red globe amaranth on tyrosinase YAN MU, LIN LI, YONG ZHOU, HAI-LIU WEI, and SONG-QING HU, Guangdong Province Key Laboratory for Green Processing of Natural Products and Product Safety, College of Light Industry and Food Sciences, South China University of Technology, Guangdong 510640, People’s Republic of China (Y.M., L.L., H.-L.W., S.-Q.H.) and School of Software Technology, Dalian University of Technology, Liaoning 116024, People’s Republic of China (Y.Z.). Accepted for publication June 19, 2012. Synopsis Tyrosinase inhibitors from natural plants are currently attracting great interest. In this study, vanillic acid (VA) from red globe amaranth fl ower was identifi ed as an effective tyrosinase inhibitor. The 50% inhibitory concentration values of VA were 0.53 and 0.63 mg/ml for the monophenolase and diphenolase activities of tyrosinase, respectively. VA did not function as a simple copper chelator, and it did not induce detectable changes in the enzyme conformation. An investigation into the interaction between VA and tyrosinase by docking method revealed that VA was bound to residues at the entrance to the dicopper center. This suggests that VA could strongly inhibit tyrosinase activity by hampering the binding of substrates to tyrosinase. Because of the stability of the complex, VA hindered binding of monophenol substrates better than that of diphenol substrates, which resulted in different inhibitory effi cacies. A study of the mechanism of tyrosinase inhibition provided new evidence to elucidate the molecular mechanism of depigmentation by red globe amaranth plant. INTRODUCTION Melanin plays an important role in skin pigmentation, and it is synthesized from tyro- sine by tyrosinase (EC 1.14.18.1). Melanin is widely distributed in nature it is found in many organisms, including microorganisms, plants and animals and catalyzes two key reactions in the melanin biosynthesis pathway: the hydroxylation of monophenol to o-diphenol (monophenolase activity) followed by the oxidation of o-diphenol to the corresponding o-quinone (diphenolase activity), which can polymerize spontaneously to form melanins (1–3). It is well documented that tyrosinase is an essential enzyme, and it is thought to be the rate-limiting enzyme in melanin synthesis. (4). Although the production of melanin in human skin is a major defense mechanism against solar irradiation, the abnormal production of the pigment can lead to melasma, freckles, Address all correspondence to Song-Qing Hu at fesqhu@scut.edu.cn.
JOURNAL OF COSMETIC SCIENCE 100 age spots, liver spots, and other types of melanin hyperpigmentation disorders, and it can cause serious aesthetic problems and even diseases such as melanoma (5). In the food in- dustry, tyrosinase activity may generate undesirable browning, which causes deleterious changes such as an unattractive appearance and reduced nutritional quality of the food product (1,6,7). In light of these cosmetic, agricultural, and medicinal problems (8), in- hibitors of tyrosinase have attracted great interest as treatments for disorders that are as- sociated with the overproduction of melanin (9). Recently, increased attention has been paid to tyrosinase inhibitors derived from natural plants, which are rich in bioactive chemicals and mostly free of side effects some of them, such as arbutin (a glycosylated hydroquinone found in certain plants), are already used in the cosmetic industry for skin whitening (10,11). The red globe amaranth, a cultivar of Gomphrena globosa, is a medicinal plant in the Amaranthaceae family. Its fl ower can be made into a highly valued scented tea. It was used to pay tribute in ancient China because of its ability to whiten skin, especially in the treat- ment of melasma, freckles, age spots, liver spots, and other forms of melanin hyperpig- mentation. Some constitutes, such as betacyanins, fl avonoids, and fl avonols, have already been isolated from some species of Gomphrena (12,13). However, the constituents of red globe amaranth plant that are responsible for whitening and the molecular mechanism of this effect are still unclear. In this study, we identifi ed for the fi rst time that vanillic acid (VA) is one of the primary skin-whitening constituents of red globe amaranth. The in- hibitory effect of VA on tyrosinase was investigated by examining enzyme kinetics, group mutations, and ability of inhibiting melanogenesis by melanocyte (14–16). However, until now, the role of the interaction between VA and tyrosinase in the inhibition of ty- rosinase remains unknown. To better understand the inhibition of tyrosinase, we analyzed the spectra and simulated the molecular interaction of VA and tyrosinase. Our study provides new evidence to help elucidate the molecular mechanism of depigmentation by red globe amaranth and helps to facilitate the proper application of this valuable plant. MATERIALS AND METHODS MATERIALS The red globe amaranth originated from Yunnan province in China and was purchased from a local Qingping herbal medicine market. L -tyrosine, L -dopa, and mushroom ty- rosinase (EC 1.14.18.1) were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were of analytical grade and manufactured in China. TYROSINASE ACTIVITY ASSAY This assay was performed using previously described methods (11) with slight modifi ca- tions. The samples were dissolved in dimethyl sulfoxide and prepared in uniform concen- trations for each batch L -tyrosine and L -dopa served as the monophenol and diphenol substrates, respectively. First, the samples were tested at only a single concentration for
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)