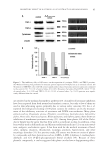
INHIBITORY MECHANISM OF RED GLOBE AMARANTH ON TYROSINASE 105 INHIBITORY EFFECT OF VA ON TYROSINASE The inhibitory effect of the prepared VA from red globe amaranth on tyrosinase activity is given in Table IV, which shows that VA had a concentration-dependent inhibitory effect on tyrosinase activity. On the basis of linear line-fi tting, the IC50 values of VA on the activities of tyrosinase monophenolase and diphenolase were 0.53 and 0.63 mg/ml, respectively. Arbutin, which is a commercial tyrosinase inhibitor, can inhibit tyrosinase monophenolase activity however, it did not inhibit diphenolase activity very well at the doses tested. VA inhibited tyrosinase activity, especially diphenolase activity, to a greater extent than arbutin when the concentration was over 0.67 mg/ml. When the dose of VA was greater than 1.00 mg/ml, the tyrosinase activity was almost inhibited. Moreover, we found that VA inhibited monophenolase activity more effectively than diphenolase activity, especially at the low concentrations of 0.17–0.67 mg/ml used in our study. Thus, VA could potentially be a good inhibitor of tyrosinase activity. VA was traced from the EA part other parts of the crude extracts also have tyrosinase inhibitory effect, such as PA and BA, although they are not the major parts, so the aban- doned parts may be the reason that the effi cacy of highly purifi ed fraction was only fi ve- fold higher than that of crude extract. EFFECT OF VA ON CONFORMATION OF TYROSINASE We investigated the structural and conformational changes caused by the addition of VA (26). The CD spectra of tyrosinase in the absence and presence of VA were investigated the result is shown in Figure 2. The CD results show that the interaction between ty- rosinase and VA hardly affects the spectra of tyrosinase in the range of 200–250 nm with two concentrations of the inhibitor, which indicates that VA interacts with tyrosinase without inducing detectable changes in the enzyme conformation. UV SPECTROSCOPIC ANALYSIS Tyrosinase is classifi ed as a type-3 copper protein family member with a dicopper (CuA and CuB) center lodged in a helical bundle. The dicopper center has a high degree of conservation and plays an important catalytic role in the activity of tyrosinase (27). When Table IV Inhibitory Effects of Vanillic Acid and Arbutin on Tyrosinase (mean value ± standard deviation) Dose (mg/ml) L -tyrosine as substrate L -dopa as substrate VA (Mean±SD) Arbutin (Mean±SD) VA (Mean±SD) Arbutin (Mean±SD) 0.17 8.19 ± 1.35 54.43 ± 0.09 2.08 ± 0.11 8.44 ± 0.77 0.33 30.70 ± 5.32 64.35 ± 1.56 3.33 ± 1.26 10.58 ± 0.68 0.67 77.16 ± 2.10 71.36 ± 2.96 59.67 ± 5.10 7.64 ± 0.93 1.00 97.74 ± 0.23 78.33 ± 0.75 82.06 ± 2.50 12.43 ± 1.27 1.33 98.83 ± 0.54 79.60 ± 1.09 90.92 ± 0.55 16.77 ± 1.73
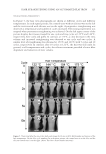
JOURNAL OF COSMETIC SCIENCE 106 inhibitors with conjugative effect interact with Cu2+, which can enhance the interior conjugation effect of the inhibitor, the spectral characteristics of the inhibitors will show red shift peaks. As a result, in research on the mechanism of tyrosinase inhibitors , UV/ visible spectroscopy can be used for studying the chelate formation between the copper ions of tyrosinase and inhibitors. Kubo and Kinst-Hori reported that kaempferol could inhibit tyrosinase activity as a copper chelator, and the inhibitory mechanism was dem- onstrated by a bathochromic shift of the spectral characteristics of kaempferol after add- ing excessive Cu2+ (21). Kim et al. found that new peaks of a fl avonoid were produced on interaction with the Cu2+ and concluded that the fl avonoid could inhibit the tyrosinase interacting with the copper ion of the enzyme (22). Figure 3 shows the changes in the UV/visible spectra of VA after the addition of tyrosinase and excess Cu2+. In the UV/vis- ible spectrum, VA had characteristic absorption bands at 255 and 288 nm, which are Figure 2. CD spectroscopy of tyrosinase with the addition of VA. Figure 3. UV/visible spectrum of VA with the addition of Cu2+ and tyrosinase.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)