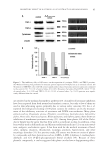
INHIBITORY MECHANISM OF RED GLOBE AMARANTH ON TYROSINASE 109 CONCLUSION As a Chinese herbal medicine, red globe amaranth strongly inhibits tyrosinase activity. In addition, we identifi ed for the fi rst time that VA is a constituent of amaranth with strong tyrosinase inhibition activity. As a potential tyrosinase inhibitor, VA could in- hibit both the monophenolase and diphenolase activities of tyrosinase better than the commercial tyrosinase inhibitor arbutin. We found that VA did not change the overall conformation of the enzyme structure and did not chelate the dicopper when it inter- acted with tyrosinase. The most probable mechanism of inhibition is that VA interacts with tyrosinase more stably than the substrates. When VA interacts with tyrosinase, the path for the substrates to the enzyme catalytic center is obstructed. Therefore, the orientation of the substrates to the dicopper center is interrupted because of the hinder- ing effect, which inhibits the monophenolase and diphenolase activities of tyrosinase. However, the interaction model and molecular mechanism were predicted according to the docking algorithm, which is limited to simulating the interaction of a protein and a ligand. Protein crystallography studies may provide further insight into the molecu- lar mechanism of inhibition. ACNOWLEDGMENTS We acknowledge the fi nanci al support from the National Natural Science Fund of China (Grant Nos 31130042 and 31171630), the Fundamental Research Funds for the Central Universities, SCUT (Grant No. 2012ZG0007), and the Program for New Century Excel- lent Talents in University (Grant No. NCET-10-0362). Dr. Wei Luo is warmly thanked for excellent technical assistance. REFERENCES (1) Y.J. Kim and H. Uyama, Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future, Cell. Mol. Life Sci., 62, 1707–1723 (2005). (2) S. Parvez, M. Kang, H.S. Chung, C. Cho, M.C. Hong, M.K. Shin, and H. Bae, Survey and mechanism of skin depigmenting and lightening agents, Phytother. Res., 20, 921–934 (2006). (3) G.M. Casanola-Martin, Y. Marrero-Ponce, M.T. Khan, A. Ather, K.M. Khan, F. Torrens, and R. Rotondo, Dragon method for fi nding novel tyrosinase inhibitors: Biosilico identifi cation and experimental in vitro assays, Eur. J. Med. Chem., 42, 1370–1381 (2007). (4) Y. Ryu, I. Westwood, N. Kang, H. Kim, J. Kim, Y. Moon, and K. Park, Kurarinol, tyrosinase inhibitor isolated from the root of Sophora fl avescens, Phytomedicine, 15, 612–618 (2008). (5) T.S. Chang, H.Y. Ding, S.S.K. Tai, and C.Y. Wu, Mushroom tyrosinase inhibitory effects of isofl avones isolated from soygerm koji fermented with Aspergillus oryzae BCRC 32288, Food Chem., 105, 1430– 1438 (2007). (6) L. Qiu, Q.X. Chen, Q. Wang, H. Huang, and K.K. Song, Irreversibly inhibitory kinetics of 3, 5-dihydroxyphenyl decanoate on mushroom (Agaricus bisporus) tyrosinase. Bioorg. Med. Chem., 13, 6206– 6211 (2005). (7) L. Qiu, Q.H. Chen, J.X. Zhuang, X. Zhong, J.J. Zhou, Y.J. Guo, and Q.X. Chen, Inhibitory effects of [alpha]-cyano-4-hydroxycinnamic acid on the activity of mushroom tyrosinase, Food Chem., 112, 609– 613 (2009). (8) K.H. Park, Y.D. Park, J.R. Lee, H.S. Hahn, S.J. Lee, C.D. Bae, J.M. Yang, D.E. Kim, and M.J. Hahn, Inhibition kinetics of mushroom tyrosinase by copper-chelating ammonium tetrathiomolybdate, Bio- chim. Biophys. Acta. Gen. Subj., 1726, 115–120 (2005). (9) P. Han, C.Q. Chen, C.L. Zhang, K.K. Song, H.T. Zhou, and Q.X. Chen, Inhibitory effects of 4-chlorosalicylic acid on mushroom tyrosinase and its antimicrobial activities, Food Chem., 107, 797–803 (2008).
JOURNAL OF COSMETIC SCIENCE 110 (10) C.K. Hsu, C.T. Chang, H.Y. Lu, and Y.C. Chung, Inhibitory effects of the water extracts of Lavendula sp. on mushroom tyrosinase activity, Food Chem., 105, 1099–1105 (2007). (11) Y.M. Chung, H.C. Wang, M. El-Shazly, Y.L. Leu, M.C. Cheng, C.L. Lee, F.R. Chang, and Y.C. Wu, Antioxidant and tyrosinase inhibitory constituents from the desugared sugarcane extract, a by-product of sugar production, J. Agric. Food Chem., 59, 9219–9225 (2011). (12) B. Dinda, B. Ghosh, S. Arima, N. Sato, and Y. Harigaya, Phytochemical investigation of Gomphrena globosa aerial parts, Incl. Med. Chem., 43, 2223–2227 (2004). (13) F. Ferreres, A. Gil-Izquierdo, P. Valentao, and P.B. Andrade, Structural characterization of phenolics and betacyanins in Gomphrena globosa by high-performance liquid chromatography-diode array detec- tion/electrospray ionization multi-stage mass spectrometry, Rapid Commun. Mass Spectrom., 25, 3441– 3446 (2011). (14) V. Kahn, N. BenShalom, and V. Zakin, Effect of benzoic acid and some of its derivatives on the rate of DL -DOPA oxidation by mushroom tyrosinase, J. Food Biochem., 21, 125–143 (1997). (15) M. Miyazawa, T. Oshima, K. Koshio, Y. Itsuzaki, and J. Anzai, Tyrosinase inhibitor from black rice bran, J. Agric. Food Chem., 51, 6953–6956 (2003). (16) T.H. Chou, H.Y. Ding, W.J. Hung, and C.H. Liang, Antioxidative characteristics and inhibition of alpha-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from Origanum vulgare, Exp. Dermatol., 19, 742–750 (2010). (17) A. Lai, M. Monduzzi, and G. Saba, Carbon-13 NMR studies on catechol, phenol and benzene deriva- tives of biological relevance, Magn. Reson. Chem., 23, 379–383 (1985). (18) C.K. Lee, C.K. Lu, Y.H. Kuo, J.Z. Chen, and G.Z. Sun, New prenylated fl avones from the roots of Ficus beecheyana, J. Chin. Chem. Soc. (Taipei, Taiwan), 51, 437–442 (2004). (19) S. Shaikh, J. Seetharamappa, P. Kandagal, and S. Ashoka, Binding of the bioactive component isothip- endyl hydrochloride with bovine serum albumin, J. Mol. Struct., 786, 46–52 (2006). (20) W. Song, M. Ao, Y. Shi, L. Yuan, X. Yuan, and L. Yu, Interaction between phillygenin and human se- rum albumin based on spectroscopic and molecular docking, Spectrochim. Acta, Part A, 85, 120–126 (2012). (21) I. Kubo and I. Kinst-Hori, Flavonols from saffron fl ower: Tyrosinase inhibitory activity and inhibition mechanism, J. Agric. Food Chem., 47, 4121–4125 (1999). (22) D. Kim, J. Park, J. Kim, C. Han, J. Yoon, N. Kim, J. Seo, and C. Lee, Flavonoids as mushroom tyrosi- nase inhibitors: A fl uorescence quenching study, J. Agric. Food Chem., 54, 935–941 (2006). (23) M. Sendovski, M. Kanteev, V.S. Ben-Yosef, N. Adir, and A. Fishman, First structures of an active bacterial tyrosinase reveal copper plasticity, J. Mol. Biol., 405, 227–237 (2010). (24) I.W. Davis and D. Baker, RosettaLigand docking with full ligand and receptor fl exibility, J. Mol. Biol., 385, 381–392 (2009). (25) J. Meiler and D. Baker, ROSETTALIGAND: Protein-small molecule docking with full side-chain fl ex- ibility, Proteins: Struct., Funct., Bioinf., 65, 538–548 (2006). (26) X. Wu, J. Liu, Q. Wang, W. Xue, X. Yao, Y. Zhang, and J. Jin, Spectroscopic and molecular modeling evidence of clozapine binding to human serum albumin at subdomain IIA, Spectrochim. Acta, Part A, 79, 1202–1209 (2011). (27) Y. Matoba, T. Kumagai, A. Yamamoto, H. Yoshitsu, and M. Sugiyama, Crystallographic evidence that the dinuclear copper center of tyrosinase is fl exible during catalysis, J. Biol. Chem., 281, 8981 (2006). (28) L.A. Kelley and M.J.E. Sternberg, Protein structure prediction on the Web: A case study using the Phyre server, Nat. Protoc., 4, 363–371 (2009). (29) E. Krissinel and K. Henrick, Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions, Acta Crystallogr., Sect D: Biol. Crystallogr., 60, 2256–2268 (2004). (30) Y.X. Si, S.J. Yin, D. Park, H.Y. Chung, L. Yan, Z.R. Lv, H.M. Zhou, J.M. Yang, G.Y. Qian, and Y.D. Park, Tyrosinase inhibition by isophthalic acid: kinetics and computational simulation, Int. J. Biol. Macromol., 48, 700–704 (2011).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)