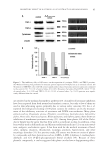
MODULATION OF CELLULAR SENESCENCE 83 changes in ATM and SA-β-Gal, while the remaining fi broblasts were cultured for an addi- tional week (approximately 2 additional passages) in the absence of test materials. For the analysis of ATM expression, at the end of the treatment period, the culture media was removed and the cells were washed once with phosphate buffered saline (PBS). After removing the wash, 1 ml of RIPA buffer (50 mM TRIS, pH 7.4, 150 mM NaCl, 1 mM PMSF, 1 mM EDTA, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 1% Triton X-100, 1% so- dium deoxycholate, 0.1% SDS) was added to the fl asks and they were incubated on ice for 15 min on a rocking platform to lyse the cells. The cell lysates were then transferred to 1.5 ml tubes and centrifuged for 5 min at 14,000 rpm (4°C). The supernatant was re- tained and stored at −75°C. The protein concentration of the supernatant was determined using a BCA Protein Assay (Pierce, Rockford, IL). ATM content was then determined using an ELISA-based assay. SA-β-Gal staining. Prior to staining, the fi broblasts were washed once with PBS and then fi xed for approximately 6 min in a fi xing solution (2% formaldehyde and 0.2% glutaral- dehyde in PBS). After fi xing, the cells were washed three times with PBS and stained using a Senescent Cells Staining Kit (Sigma-Adrich, St. Louis, MO) per the kit’s instruc- tions. The cells were then incubated at 37°C overnight in a non-CO2 incubator. On the following day, the staining solution was removed and replaced with PBS. The cells were then photographed microscopically, and the number of stained cells (SA-β-Gal positive) in each fi eld was counted. Dermal papillae studies. Human dermal papillae cells were seeded into 12 plates in dermal papillae growth well medium (DPGM) and grown at 37 + 2°C and 5 + 1% CO2 until confl uent with a media change every 48 to 72 h as needed. Once the cells were confl uent, the cell culture media was replaced with PBS and the cells were irradiated with 20 mJ/cm2 UVB. After the UVB irradiation, the PBS was removed and replaced with cell culture media supplemented with the various test materials. Nonsupplemented DPGM was used as the untreated control. In addition, one set of cells was not exposed to UVB and served as the non-UVB treated control. After the addition of the media, sets of cells were cul- tured for 48 h. At the end of the incubation period, the cells were assayed for changes in SA-β-Gal activity as described above. Statistical analysis. Treatments were compared via an ANOVA with a subsequent post hoc analysis (Newman–Keuls Multiple Comparison) using Graphpad Prism Software. Statis- tical signifi cance was set at p 0.05. RESULTS AND DISCUSSION GENOMIC RESPONSE OF HEXAPEPTIDE-11 ON NORMAL HUMAN FIBROBLASTS The purifi ed hexapeptide was initially examined for cytotoxic effects on normal human dermal fi broblasts. No cytotoxic effects were noted up to 1.0% of hexapeptide treatment (data not shown). Hexapeptide-11 was then evaluated at 0.001% and 1.0% concentra- tions on normal human dermal fi broblasts for 24 h by gene microarray analysis to deter- mine the biological effect that Hexapeptide-11 has ATM gene expression (Figure 2). Both concentrations demonstrated statistically signifi cant reductions in ATM gene ex- pression as shown by their corresponding ratio of medians being less than 0.7 (25).
JOURNAL OF COSMETIC SCIENCE 84 INFLUENCE OF HEXAPEPTIDE-11 ON STRESSED-INDUCED PREMATURELY SENESCENT FIBROBLAST CELLS Premature cellular senescence can be induced by treating normal human dermal fi bro- blasts with H2O2 (26). The hexapeptide delays senescence in H2O2 stress-induced prematurely senescent dermal fi broblasts as measured by ATM protein expression in a dose-dependent fashion at peptide concentrations of 1.0% or more, but not at levels 0.1% and below (Figure 3). Interestingly, the infl uence of the peptide on ATM expression at concentrations greater than 1% does not continue to show increasing suppression of ATM protein expression, but instead shows a leveling effect up to 2% of hexapeptide concentration. The reasons for this leveling effect are unknown at this time, but additional studies reported here were typically run at concentrations no greater than 1.0% because of these initial dose fi ndings. To examine the infl uence of Hexapeptide-11 on intrinsic aging, normal human dermal fi broblasts were grown through 24 population doublings without additional added oxi- dative stress. Topical application of Hexapeptide-11 for the last 18 cycles showed a dose-dependent, statistically signifi cant reduction of both ATM protein expression and SA-β-Gal expression, Figures 4 and 5, respectively. The effect on ATM protein and SA- β-Gal expression was reversible after 1 week of peptide removal indicating that infl uence of the hexapeptide on these cellular markers is not permanent. Figure 3. Changes in ATM protein expression in H2O2 prematurely stressed normal human dermal fi bro- blasts treated with various concentrations of Hexapeptide-11. Figure 2. Summary of ratio of medians for two concentrations of Hexapeptide-11, 0.001% and 1.0% for ATM expression after 24 h treatment on normal human dermal fi broblasts.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)