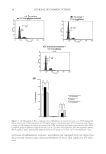
J. Cosmet. Sci., 65, 1–9 ( January/February 2014) 1 Comparison of damage to human hair fi bers caused by monoethanolamine- and ammonia-based hair colorants AARON D. BAILEY, GUIRU ZHANG, and BRYAN P. MURPHY, Procter & Gamble, Beauty Technology Division, Mason Business Center, Mason, OH 45040. Accepted for publication July 17, 2013. Synopsis The number of Level 3 hair color products that substitute 2-aminoethanol [monoethanolamine (MEA)] for ammonia is increasing. There is some anecdotal evidence that higher levels of MEA can be more damaging to hair and more irritating than a corresponding equivalent level of the typical alkalizer, ammonia (in the form of ammonium hydroxide). Our interest was to understand in more quantitative terms the relative hair damage from the two alkalizers, particularly at the upper limits of MEA on-head use. Limiting investigations of oxidative hair damage to increases in cysteic acid content (from cystine oxidation) can underreport the extent of total damage. Hence, we complemented Fourier transform infrared spectroscopy (FTIR) cysteic acid level measurement with scanning electron microscopy (SEM) photomicrographs to visualize cuticle damage, and protein loss to understand not only the oxidative damage but also the damage caused by other damage pathways, e.g., reaction of the more nucleophilic (than ammonia) MEA with hair protein. In fact, all methods show an increase in damage from MEA-based formulations, up to 85% versus ammonia in the most extreme case. Hence, if the odor of ammonia is a concern, a better approach may be to minimize the volatility of am- monia in specifi c chassis rather than replacing it with high levels of a potentially more damaging alkalizer such as MEA. INTRODUCTION Alkalizers have been key components of Level 3 oxidation dyes since these products were developed (1). Typically it was ammonia, which has been the industry standard because of its effectiveness. Alkalizers serve three important functions: swell the hair fi ber to al- low better penetration of dye precursors, generate the active peroxide species necessary for melanin bleaching and dye formation, and participate in the bleaching of melanin (2). Because of the characteristic odor of ammonia, other alkalizing agents have been used as replacements, particularly where extreme lightening is not necessary. Although commercial versions of these alternate alkalizers also have characteristic odors, some people fi nd them less objectionable than the odor of ammonia. For example, 2-amino-2-methylpropanol has been used in Level 2 oxidative hair color products. In addition, Level 3 lift was Address all correspondence to Bryan Murphy at Murphy.bp.1@pg.com
JOURNAL OF COSMETIC SCIENCE 2 achieved for the fi rst time with a high level of MEA in Clairesse® (Proctor & Gamble, Stamford, CT), which was launched by Clairol in 1981. Later, Herbal Essences® Hair Color from Clairol (Proctor & Gamble) used a lower level of monoethanolamine (MEA) to achieve many Level 3 shades, and achieved the higher lift shades by adding a small amount of ammonia. MEA also was used successfully in Level 2 products such as Cast- ing® from L’Oréal (Clichy CEDEX France) and Natural instincts® from Clairol, in which less lift is required than for a Level 3 hair colorant. One of the challenges of using MEA in hair color formulations is that an increased per- centage (relative to ammonia) is required to generate the same level of lightening (bleach- ing, lift) of the hair’s melanin (2). This is of particular concern, because Seo et al. (3) have observed synergistic causality of dermatitis and hair loss by higher levels of MEA and hydrogen peroxide. In addition to smell, one of the key concerns with Level 3 colorants is the amount of dam- age that is done to the hair fi ber, either during a single use or repetitive uses as the hair is re-colored. This is particularly true in the salon environment where consumers tend to use the same colorant product for extended periods rather than switching among prod- ucts. Damage can be masked or repaired with hair treatment agents for both MEA- and ammonia-based products, or mitigated in ammonia-based products with radical scaven- gers (4) or chelants (5), but starting with ingredients that cause the least damage makes damage mitigation easier. Among other components, hair is composed of proteins and lipids that are susceptible to a variety of chemical reactions such as oxidation and nucleophilic attack. Of course, the observed rates of these attacks are dependent on a variety of factors such as concen- trations, pH, and the individual rate constants. Ammonia is nucleophilic, but less so than MEA. For example, MEA is well known to be nucleophilic enough to be a key reagent in the synthesis of dyes through SNAr reactions (6) and the removal of ester- protecting groups from air-sensitive coloring agents (7). Whereas excess ammonia quickly leaves the hair because of its volatility, MEA is not volatile under atmospheric conditions, so there is the potential for damage to be exacerbated over time if signifi - cant amounts of MEA remain in the hair after rinsing. Our interest was whether there are any differences between ammonia and MEA in the extent of damage to hair fi bers when they are used at the concentrations needed to get enough bleaching for Level 3 oxidation dye products. GENERAL EXPERIMENTAL MATERIALS Chemicals used in the bleaching chassis were of the grades commonly used in cosmetic products and were used as received. Cetearyl alcohol was purchased from Cognis Corp. (Monheim, Germany), Crodafos CES® and steareth-200 from Croda Chemicals Europe Ltd. (East Yorkshire, England), xanthan gum from CP Kelco (Atlanta, GA), sodium hy- droxide from Brenntag GMBH (Mulheim, Germany), sodium sulfate from Cordenka GmbH (Obernburg, Germany), sodium sulfi te from Esseco SRL (Novara, Italy), ascorbic acid from DSM Nutritional (Kaiseraugst, Switzerland), ethylenediaminetetraacetate di- sodium salt from Akzo Nobel Surface Chemistry Inc. (Amsterdam, Netherlands), propylene
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)