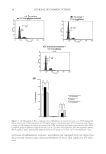
EFFECTS OF TAMARIND SEED COAT EXTRACT ON HUMAN SKIN 17 amount of cell damaged caused through H2O2 by 25–45%. The preventive action of the extract may occur from free-radical scavenging activity of polyphenols contained in the extract. Antioxidative properties of polyphenols arise from their high reactivity as hydro- gen donors or electron acceptors and from the ability of the polyphenol-derived radical to stabilize and delocalize the unpaired electron (28). Another possible action underlying the antioxidative properties of the extract is the ability of polyphenols to alter peroxida- tion by modifi cation of the lipid packing order and to decrease membranes fl uidity (29). These changes can sterically hinder diffusion of free radicals and restrict peroxidative re- actions. These actions can protect cell damage from membrane lipid peroxidation and apoptosis. PREVENTIVE EFFECT OF THE EXTRACT TO UVA-INDUCED CELL ALTERATIONS UVA radiation has long been known to generate an oxidative stress in cells irradiated in culture, and this has been linked to many studies that show that both endogenous anti- Figure 2. Effect of tamarind seed coat extract on viability of human skin fi broblasts. Data are expressed as percentage of control (untreated cells), and each bar represents mean ± SD of triplicate study. Figure 3. Preventive effect of tamarind seed coat extract on cell damage in H2O2-induced fi broblasts. Data are expressed as percentage of cell damage relative to 0% damage calculated from the number of cells that were not exposed to H2O2, and each bar represents mean ± SD of triplicate study. Signifi cantly different from the untreated group **p 0.01 (Student’s t-test).
JOURNAL OF COSMETIC SCIENCE 18 oxidant pathways and added antioxidants can protect against the damage that arises from exposure to UVA (30–33). Endogenous antioxidant defense molecules such as glutathione are depleted in skin and skin cells by UVA radiation and this may lead not only to apoptosis in human keratinocytes (34) but also to the induction of the interstitial collagenase, MMP-1, in cultured skin fi broblasts (35,36). This study investigated the preventive effects of tamarind seed coat extract on skin fi broblasts after UVA irradiation, in terms of levels of MMP-1, type I procollagen, and antioxidant defense molecules such as total glutathione. The XTT assay showed that viability of fi broblasts was decreased (∼25%) at 24 h after UVA exposure. However, this was almost completely reversed by the extract at concentra- tion used (200 μg/ml) (Fig. 4B). Under UVA, many of the fi broblasts became more rounded whereas cultures pretreated with extract showed a greater proportion of spindle- shaped fi broblasts resembling the controls (Fig. 4A). We then assessed more subtle changes in the cultured fi broblasts by studying their replicative ability after radiation. After radiation, there was a clear induction in the number of cells in the arrested G0/G1 phase (from 59% to 78%, p 0.01) (Fig. 5) and a corresponding reduction in the G2/M (mitotic) phase (from 16% to 9%, p 0.05). The obtained results coincide with the pre- vious study indicating increase in G0/G1 proportion and decrease in G2/M proportion in low-dose UVA-irradiated fi broblasts (37). The arrest in the G0/G1 suggests that fi bro- blasts were in the resting state cell synthesis and proliferation were inhibited. Interest- ingly, pretreatment of UV-irradiated cells with the extract showed a cell-cycle profi le very similar to the nonirradiated controls. These observations indicate that tamarind seed coat extract is effective in the prevention of UVA-induced fi broblast cell arrest in G0/G1. Fur- ther studies should be performed to clarify preventive mechanism of the extract, particu- larly a p53-dependent cell-cycle arrest. Glutathione is an important cell antioxidant and crucial in the regeneration of other en- dogenous antioxidants, and thus its level is a sensitive indicator of oxidative stress (38,39). In healthy cells, ∼95% of the intracellular glutathione is present in its reduced form (GSH) because of an effi cient pathway to re-reduce the oxidized form (GSSG). After 6 h of UVA irradiation, total glutathione of UVA-irradiated group was markedly increased to 25% (p 0.05), compared to the control that was set 100% (Fig. 6). This implies that one of the cellular responses against UV exposure causing high-level ROS is the induc- tion of detoxifying system level. However, the accumulation of ROS resulted in low level of total glutathione as a result in 72 h incubation of UVA-irradiated group. Interestingly, pretreatment with the extract restored the level of total glutathione to the level found in the control group. The extract may suppress the formation of radicals and protect against cell damages and/or function alterations. Moreover, the extract may have capacity to im- prove the activity of glutathione and/or increase the level of intracellular glutathione. The mechanism underlying the extract-induced total glutathione level/activity is needed to clarify in the future. Crucial functions of skin fi broblasts are the biosynthesis and secretion of type 1 procol- lagen and MMP-1, and these were measured in the cell supernatant by enzyme immu- noassay at 24, 48, and 72 h after UVA irradiation. There was a clear increase in secretion of MMP-1, which had normalized at 72 h after radiation (Fig. 7A). In contrast, the extract prevented this increased MMP-1 secretion. In contrast to MMP-1, radiation had essentially the reverse effect on procollagen biosynthesis (Fig. 7B). However, this
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)