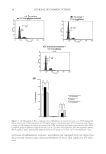
MOLECULAR MARKER APPROACHES FOR TRACKING REDOX DAMAGE 35 Both wool damage marker peptides, SFGYR and DVEEWYIR, underwent signifi cant photooxidative modifi cation under UVA and UVB irradiation, evidenced by decreases in the abundance of MH+ ions at m/z 629.3 and 1109.6, and corresponding increases in the relative abundance of their oxidized products. The relative increases of SFGY*R Y+O, Y+2O, and Y+3O and of DVEEYW*IR W+O and W+2O provided an indication of the level of specifi c protection conferred by the treatment. Oxidation of the two native marker peptides was minimized in the samples with solgel surface treatment. In addition to demonstrating the UV-protective effect of a silicon-based solgel matrix surface coating for wool at the molecular level, this evaluation validated the utility of marker peptides for characterizing and tracking redox damage and the effects of protec- tive treatments on these factors, even within a complex proteinaceous system. CONCLUSIONS This study has demonstrated and validated the utilization of marker peptides for profi ling and tracking redox modifi cation, as well as evaluating and validating protection or repair treatments. These are two valuable goals for hair and skin research and development. Six marker peptides, fi ve containing tyrosine and one containing tryptophan, were identi- fi ed and validated through the evaluation of light-induced oxidative damage in an en- riched keratin preparation. A full redox proteomic evaluation of UVA photomodifi cation to aromatic residues in whole wool was successfully performed, with mapping of a wide range of modifi cations through the wool proteome. Subsequently, two selected keratin marker peptides, SFGYR and DVEEWYIR, were utilized both to track oxidative modi- fi cation in whole wool through a range of UV irradiation protocols and to demonstrate the UV-protective effect of a prior solgel surface treatment of wool at the molecular level. The utilization of such marker peptides has the potential for a range of cosmetic applications in hair, skin, and nails, in addition to applications tracking redox modifi cation in protein foods and biomaterials. We anticipate that further development of this redox proteomic marker ap- proach will allow more targeted design and evaluation of protection and repair treatments for protein systems, through mapping the type and location of damage at the molecular level. ACKNOWLEDGMENTS We gratefully acknowledge support for this work from Wool Research Inc. (05WRCN03/08WRAG01) and the New Zealand Foundation for Research Science and Technology (C10X0710). REFERENCES (1) D. S. Fudge, T. Winegard, R. H. Ewoldt, D. Beriault, L. Szewciw, and G. H. McKinley, From ultra-soft slime to hard {alpha}-keratins: The many lives of intermediate fi laments, Integr. Comp. Biol., 49, 32–39 (2009). (2) C. Popescu and H. Höcker, “Chapter 4: Cytomechanics of Hair: Basics of the Mechanical Stability,” in International Review of Cell and Molecular Biology, W. J. Kwang. Ed. (Academic Press, London, 2009), pp. 137–156.
JOURNAL OF COSMETIC SCIENCE 36 (3) K. R. Millington and J. S. Church, The photodegradation of wool keratin II. Proposed mechanisms involving cystine, J Photochem Photobiol B., 39, 204–212 (1997). (4) R. H. Bradley, I. L. Clackson, and D. E. Sykes, UV ozone modifi cation of wool fi bre surfaces, Appl Surf Sci, 72, 143–147 (1993). (5) D. Yilmazer and M. Kanik, Bleaching of wool with sodium borohydride, J Eng Fiber Fabr., 4, 45–50 (2009). (6) J. M. Dyer, J. Plowman, G. Krsinic, S. Deb-Choudhury, H. Koehn, K. Millington, et al., Proteomic evaluation and location of UVB-induced photo-oxidation in wool, J Photochem Photobiol B., 98, 118–127 (2010). (7) J. M. Dyer, S. Bringans, and W. G. Bryson, Characterisation of photo-oxidation products within pho- toyellowed wool proteins: Tryptophan and tyrosine derived chromophores, Photochem Photobiol Sci., 5, 698–706 (2006). (8) B. Mahltig, F. Audenaert, and H. Bottcher, Hydrophobic silica sol coatings on textiles—The infl uence of solvent and sol concentration, J Sol-Gel Sci Technol., 34, 103–109 (2005). (9) Technical release: Luzchem exposure standard: LES-UVA-01 (2004) Available from: http://www. luzchem.com/handbook/LESUVA011.pdf. (10) Technical release: Luzchem exposure standard: LES-UVB-01 (2004) Available from: http://www. luzchem.com/handbook/LESUVB011.pdf. (11) Technical release: Luzchem exposure standard: LES-420-01 (2004) Available from: http://www.luzchem. com/handbook/LES420011.pdf. (12) H. Thomas, A. Conrads, K. H. Phan, M. van de Löcht, and H. Zahn, In vitro reconstitution of wool intermediate fi laments, Int J Biol Macromol., 8, 258–264 (1986). (13) N. R. Parker, J. F. Jamie, M. J. Davies, and R. J. W. Truscott, Protein-bound kynurenine is a photosen- sitizer of oxidative damage, Free Radic Biol Med., 37, 1479–1489 (2004). (14) A. Pirie, Formation of N′-formylkynurenine in proteins from lens and other sources by exposure to sunlight, Biochem J., 125, 203–208 (1971). (15) T. Gensch, J. Hendriks, and K. J. Hellingwerf, Tryptophan fl uorescence monitors structural changes accompanying signalling state formation in the photocycle of photoactive yellow protein, Photochem Photobiol Sci., 3, 531–536 (2004). (16) J. Lee, N. Koo, and D. B. Min, Reactive oxygen species, aging, and antioxidative nutraceuticals, Compr Rev Food Sci Food Saf., 3, 21–33 (2004). (17) C. Wei, B. Song, J. Yuan, Z. Feng, G. Jia, and C. Li, Luminescence and Raman spectroscopic studies on the damage of tryptophan, histidine and carnosine by singlet oxygen, J Photochem Photobiol A Chem., 189, 39–45 (2007). (18) J. A. Maclaren and B. Milligan, “The Structure and Composition of Wool,” In Wool Science—The Chem- ical Reactivity of the Wool Fibre (Science Press, Marrickville, Australia, 1981), pp. 1–18. (19) R. C. Marshal, D. F. G. Orwin, and J. M. Gillespie, Structure and biochemistry of mammalian hard keratin, Electron Microsc Rev., 4, 47–83 (1991).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)