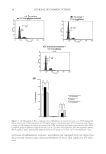
COMPARISON OF MONOETHANOLAMINE AND AMMONIA HAIR COLORANTS 7 of treatments for a particular alkalizer. Cysteic acid content really did not break out large differences between ammonia- and MEA-based chassis, and better measures were needed. SEM photomicrographs were obtained to complement the cysteic acid values because it is known that cuticle, where the highest percentage of cystine resides, is removed some- what during chemical treatments and mechanical abrasion. Although the increase in damage as a function of increasing concentrations of alkalizers and increasing numbers of treatments is as we would expect, i.e., more treatments generate more damage, we wanted to ensure that we had a complete damage assessment, because if cuticle is removed, the cysteic acid value may be misleading. Figures 1 to 3 show SEM images for hair treated with 0.27 M, 0.54 M, and 0.82 M alkalizer. Note that cuticle stripping, and in some cases complete removal of the cuticle, increases as the concentration on MEA is increased. Although there is damage caused by the am- monia formulation, it is less so than for the MEA formulation. Figures 1 to 3 show that in each case, MEA causes more cuticle lifting and removal than the corresponding ammonia formulation at the same molar concentration. Figure 2. SEM photomicrographs obtained on a Hitachi S-3000N SEM with Oxford detector after the fi fth bleaching cycle of light brown hair treated with formulations containing 0.54 M ammonia (A) and MEA (B) for 30 min at 30°C. Figure 3. SEM photomicrographs obtained on a Hitachi S-3000N SEM with Oxford detector after the fi fth bleaching cycle of light brown hair treated with formulations containing 0.82 M ammonia (A) and MEA (B) for 30 min at 40°C.
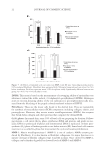
JOURNAL OF COSMETIC SCIENCE 8 Figure 4. Traditional, soluble, and insoluble protein fractions of hair treated with a 0.82 M MEA/3% H2O2 formulation (30°C) or a 0.82 M NH3/3% H2O2 formulation (30°C), measured with the modifi ed Lowry method kit (8). Given that both Fourier transform infrared spectroscopy (FTIR) cysteic acid analysis and SEM show the most damage for the 0.82 M alkalizer concentration, we performed protein loss analysis on this concentration of both the ammonia and the MEA formulations (Figure 4). Protein loss analysis complements the cysteic acid method that measures oxidative dam- age. It enables us to quantify damage that may be due to nucleophilic attack on the kera- tin fi bers by species generated by peroxide (e.g., –OOH), −OH, NH3, or MEA, as well as the oxidative damage. Naqvi et al. (9) have shown that their method to measure protein loss, which quantifi es soluble and insoluble protein separately, provides a better under- standing of the form in which protein is lost and is a more accurate representation. Tra- ditional protein loss method measurements are included to demonstrate that the observed effect of increased damage by MEA-containing formulations is not a function of the method. Samples of hair were treated once with either a 0.82 M MEA or a 0.82 M am- monia formulation (6% H2O2 30°C). The tresses were rinsed and shampooed once by the procedure described earlier. By the traditional method (measuring both soluble and insoluble proteins together), we demonstrate that the MEA-based formulation generates 40% more protein loss (dam- age) than the standard ammonia formulation. Similarly, soluble and insoluble protein losses show 58% and 85% more damage, respectively. This is in keeping with the fi nd- ings of the original reference, which shows that the traditional protein loss measurement method generally underrepresents the total protein loss. Clearly, the use of MEA rather than ammonia at 0.82 M (5% by weight), a common con- centration of MEA used for higher levels of lift in Level 3 products, results in more pro- tein loss (damage) than an ammonia-based product, even after only one treatment. CONCLUSIONS There are trade-offs for less odor in Level 3 hair color: performance and damage. The trade- off in performance (extent of lightening at maximum accepted alkalizer concentration) is
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)