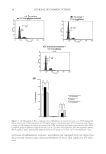
ANALYSIS OF DISTRIBUTION OF WATER IN HAIR 47 refl ection in the diffraction pattern is attributed to the organization of the protofi laments in the cortical cells of hair. Amount of water in these regions of IFs appears to be relatively small compared to the total amount. A larger fraction of water appears to be present in the mesopo- rous regions, as indicated by the high-intensity near-zero scattering angles. Obvious features that correspond to the mesoporous entities in hair can be associated with the medullary struc- tures, which are likely to be more hydrophilic and thus interact positively with water. Other mesoporous entities are the cracks generated by fi ber damage, both in the cortex and the en- docuticle region of the cuticle cell. Further SANS experiments with carefully prepared and well-characterized hair samples and analysis of the data could provide a detailed distribution of water in regions other than the IFs. Figure 7. IR spectra of hair. (A) Exposed to D2O, and then (B) immersed in boiling water.
JOURNAL OF COSMETIC SCIENCE 48 ACKNOWLEDGMENTS These data were collected at beam lines CG-2 and CG-3 at the High Flux Isotope Reactor (HFIR) at the Oak Ridge National Laboratory. We thank Dr William Heller at HFIR for enabling the collection of the neutron scattering data, and Dr Wenjie Wang for process- ing some of the 2D data used in the fi gures. REFERENCES (1) E. Fuchs and D.W. Cleveland, A structural scaffolding of intermediate fi laments in health and disease, Science, 279, 514–519 (1998). (2) C.R. Robbins, Chemical and Physical Behavior of Human Hair. (Springer-Verlag, New York, 1994). (3) P. Jolles and H. Zahn, Hair: Biology and Structure. Vol. 78. (Birkhäuser, Basel, 1996). (4) M. Feughelman, A two-phase structure for keratin fi bers, Text. Res. J., 29, 223–228 (1959). (5) C. Keis, C.L. Huemmer, and Y.K. Kamath, Effect of oil fi lms on moisture vapor absorption on human hair, J. Cosmet. Sci., 58, 135–146 (2007). (6) G.D. Wignall, “Small-angle-neutron-scattering characterization of polymers,” in Physical Properties of Polymers. (Cambridge University Press, Cambridge, U.K., 1993), pp. 424–511. (7) N.S. Murthy, M. Stamm, J.P. Sibilia, and S. Krimm, Structural changes accompanying hydration in nylon 6, Macromolecules, 22, 1261–1267 (1989). (8) J. Blazek and E.P. Gilbert, Application of small-angle X-ray and neutron scattering techniques to the characterisation of starch structure: A review, Carbohydr. Polym., 85, 281–293 (2011). (9) N.S. Murthy, Fibrillar structure and its relevance to diffusion, shrinkage, and relaxation processes in nylon fi bers1, Text. Res. J., 67, 511–520 (1997). (10) D.T. Grubb and N.S. Murthy, Real-time X-ray study of nylon-6 fi bers during dehydration: Equatorial small-angle scattering is due to surface refraction, Macromolecules, 43, 1016–1027 (2009). (11) N.S. Murthy and W.J. Orts, Hydration in semicrystalline polymers: Small-angle neutron scattering stud- ies of the effect of drawing in nylon-6 fi bers, J. Polym. Sci. Part B: Polym. Phys., 32, 2695–2703 (1994). (12) M.E. Rafi k, F. Briki, M. Burghammer, and J. Doucet, In vivo formation steps of the hard α-keratin interme- diate fi lament along a hair follicle: Evidence for structural polymorphism, J. Struct. Biol., 154, 79–88 (2006). (13) F. Briki, B. Busson, B. Salicru, F. Estève, and J. Doucet, Breast-cancer diagnosis using hair, Nature, 400, 226 (1999). (14) V. James, J. Kearsley, T. Irving, Y. Amemiya, and D. Cookson, Using hair to screen for breast cancer, Nature, 398, 33 (1999). (15) J.L. Leveque, J.C. Garson, P. Pissis, and G. Boudouris, Free water in hair keratin? A depolarization thermal-current study, Biopolymers, 20, 2649–2656 (1981). (16) W. Wang, N.S. Murthy, and D.T. Grubb, Central small-angle diffuse scattering from fi bers is made of two components, J. Polym. Sci. Part B: Polym. Phys., 50, 797–804 (2012). (17) R.G. Quynn, Internal volume in fi bers, Text. Res. J., 33, 21–34 (1963). (18) P.H. Emmett, Adsorption and pore-size measurements on charcoals and whetlerites, Chem. Rev., 43, 69–148 (1948). (19) Y.Z. Hessefort, B.T. Holland, and R.W. Cloud, True porosity measurement of hair: A new way to study hair damage mechanisms, J. Cosmet. Sci., 59, 303–315 (2008). (20) R. De Cassia Comis Wagner, P.K. Kiyohara, M. Silveira, and I. Joekes, Electron microscopic observa- tions of human hair medulla, J. Microsc., 226, 54–63 (2007). (21) R.L. McMullen and S.P. Kelty, Investigation of human hair fi bers using lateral force microscopy, Scan- ning, 23, 337–345 (2001). (22) S. Nagase, S. Shibuichi, E. Kariya, N. Satoh, and K. Ando, Infl uence of internal structures of hair fi ber on hair appearance. I. Light scattering from the porous structure of the medulla of human hair, J. Cosmet. Sci., 53, 89–100 (2002). (23) S.B. Ruetsch, Y.K. Kamath, A.S. Rele, and R.B. Mohile, Secondary ion mass spectrometric investiga- tion of penetration of coconut and mineral oils into human hair fi bers: Relevance to hair damage, J. Cosmet. Sci., 52, 169–184 (2001). (24) S.B. Hornb y, Y. Appa, S.Ruetsch, and Y. Kamath, Mapping penetration of cosmetic compounds into hair fi bers using time of fl ight secondary ion mass spectroscopy (TOF–SIMS), IFSCC Magazine, 8, 99– 104 (2005).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)