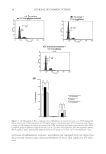
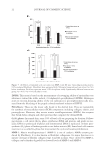
COMPARISON OF MONOETHANOLAMINE AND AMMONIA HAIR COLORANTS 3 glycol from Lyondell Chemical Co. (Houston, TX), trisodium ethylenediamine disucci- nate from Innospec CTD (Littleton, CO), ammonia from EMD (Darmstadt, Germany), and monoethanolamine from Huntsman Corp. (The Woodlands, TX). Deionized water was used to prepare the chassis. Hair (blended source light brown virgin hair) was purchased from International Hair Importers & Products. Hair treatments were performed with Pantene® Fine Hair Solu- tions Flat to Volume shampoo and Pantene® Classic Conditioner (Procter & Gamble, Cincinnati, OH). The developer used 20 volume Welloxon® (Procter & Gamble, Cincin- nati, OH), which corresponds to 6% by weight hydrogen peroxide. METHODS Preparation of Chassis Part 1. To a 10 l vessel was added Crodafos CES® (847.5 g), cetearyl alcohol (45.5 g), and steareth-200 (81 g). The mixture was heated to 85°C. Water (3.63 kg) and sodium hydroxide (34 g) were added during heating and the mixture was homoge- nized for 1 min at 15 m/s. After the mixture is completely homogenized, agitation was performed for 10 min at 1.1 m/s while heating was continued. To a separate beaker was added xanthan gum (15 g) and propylene glycol (49 g), which was mixed until it was homogeneous. The gum–glycol mixture was then added to the Crodafos CES® mixture, which was 80°C. The combined mixture was then homogenized for 1 min at 20 m/s and then agitated for 10 min at 1.1 m/s. The batch was transferred to large mixing vessel once the temperature reached 85°C. The batch was thoroughly homogenized at 20 m/s for 5 min. Hot water (500 g) was then added to the batch, which then was subjected to cooling under vacuum with stirring at 0.6 m/s. When the temperature decreased to 50°C, cool- ing was stopped. Preparation of ammonia and monoethanolamine bleaching chassis. To a dry 2 l Griffi n beaker was added Chassis Part 1 (257.5 g). To a separate 450 ml beaker was added sodium sulfate (5.0 g), sodium sulfi te (2.0 g), ascorbic acid (1.5 g), ethylenediaminetetraacetate diso- dium salt (0.5 g), propylene glycol (37.5 g), and water (amount indicated in Table I). The mixture was heated and stirred at 55°C until all salts were dissolved. The solution was then added slowly with stirring (overhead stirrer with 4 paddles ∼270 rpm) to the beaker containing Chassis Part 1. On complete addition, trisodium ethylenediamine disuccinate Table I Amounts of Formulation Ingredients Required to Prepare the NH3 and Monoethanolamine (MEA) Bleaching Chassis. Ingredients That Do Not Vary in Concentration Are Given in the Experimental Procedure Alkalizer after mixing with H2O2 developer 25% Aqueous NH3 (g) MEA (g) Water (g) pH after mixing 1:1 with H2O2 0.27 M NH3 18.18 0 155.75 10.11 ± 0.02 0.54 M NH3 36.41 0 139.2 10.35 ± 0.02 0.82 M NH3 55.76 0 117.9 10.47 ± 0.02 0.27 M MEA 0 16.3 155.75 10.24 ± 0.02 0.54 M MEA 0 32.65 146.6 10.42 ± 0.02 0.82 M MEA 0 50 129.25 10.6 ± 0.02
JOURNAL OF COSMETIC SCIENCE 4 (16.75 g of a 32 wt% solution in water) was added dropwise with stirring, and the resulting mixture was stirred for 10 min at which time 25% aqueous NH3 or MEA (amount indicated in Table I) was added. Water was added so that total weight equaled 500 g. The resulting mixture was stirred for 10 min. Procedure for bleaching tress and rinse/wash cycle. To a plastic weigh boat was added 3 g of dyeless chassis and 3 g of 20 volume Welloxon® developer (6% H2O2). The two gels were mixed thoroughly and the pH was measured [Mettler Toledo SevenEasy™ pH meter S20 (Columbus OH)] before applying to the desired 1.5 g hair tress. After uni- formly applying the mixture to the tress, the hair was placed in a covered weigh boat and incubated in a 30°C oven for 30 min. After completion of the incubation period, the hair was removed from the oven and rinsed for 2 min (37 ± 2°C water at a fl ow rate of 1.0 ± 0.2 gal/min), shampoo (0.15 ml per tress) was added and massaged into the hair for 30 s followed by a 30-s rinse (massaging hair once in every 2 s). On completion, the hair was dried using a blow dryer on high heat/high air fl ow for 3 min (1 min per side + 1 min combing). Shampoo/conditioner cycles. The tresses were subjected to additional treatments with Pantene® Fine Hair Solutions Flat to Volume shampoo and Pantene® Classic Conditioner. One complete cycle was performed as follows: • The hair was wetted (37 ± 2°C water at a fl ow rate of 1.0 ± 0.2 gal/min) for 30 s. • Shampoo (0.1 ml/g hair) was applied and the hair was massaged thoroughly for 30 s. • The hair was rinsed (massaging every other second) for 30 s, blotted with a towel, and blown dry (high heat/high air fl ow) for 3 min (1 min each side with fi nger pressing fi nal minute brushing with hair brush). • The hair was again wetted (37 ± 2°C water at a fl ow rate of 1.0 ± 0.2 gal/min) for 30 s. • Shampoo (0.1 ml/g hair) was applied and the hair was massaged thoroughly for 30 s. • The hair was rinsed (massaging every other second) for 30 s. • Immediately after the second shampoo rinsing, conditioner (0.1 ml/tress 1.5 g tresses) was applied for 30 s and rinsed for 30 s (massaging every 2 s). • The hair was patted dry with a towel before being blown dry (high heat/high air fl ow) for 3 min (1 min each side with fi nger pressing fi nal minute brushing with hair brush). The above steps were repeated an additional 17 times for a total of 18 cycles after each bleaching treatment. Cysteic acid determination. Cysteic acid readings were obtained on a Perkin Elmer Spectrum 100 FT-IR (Waltham MA) with universal attenuated total refl ectance (UATR) sampling accessory immediately following bleaching as well as after the wash cycles. The hair tress was fastened onto the baseplate of the UATR and was twisted three rotations, then 70 N was applied by means of a piston. The collected absorbance spectrum is normalized by setting the highest peak between 1000 and 2000 cm−1 to 1.5 AU. The invariant 1450 cm−1 peak is set to zero AU and the second derivative of the normalized spectrum is taken. The second derivative of the 1040 cm−1 peak (cysteic acid, S=O stretch) is multi- plied by −104 to give the cysteic acid value. Four measurements were taken and the arithmetic mean of those measurements was calculated to give the output reading. The process was performed on four locations along the length of the hair tress. Values reported
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)