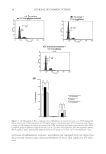
EFFECTS OF TAMARIND SEED COAT EXTRACT ON HUMAN SKIN 13 and phosphotungstate used for the colorimetric assay of phenolic antioxidants and poly- phenol antioxidants. The reagent reacts with phenols and nonphenolic reducing sub- stances to form chromogens that were measured spectrophotometrically. An aliquot of the samples (40 μl) was mixed with 1.8 ml Folin–Ciocalteu reagent previ- ously diluted with distilled water (1:10). The solution was incubated at 25oC for 5 min before adding 1.2 ml of 15% sodium carbonate solution in distilled water. After incuba- tion at room temperature for 30 min, the absorbance at 760 nm was measured. The amount of total phenolic compounds was calculated as gallic acid equivalents from the calibration curve. The result was shown in gallic acid equivalents (g/100 g sample). FREE RADICAL SCAVENGING ACTIVITY ON DPPH The antioxidant activity of tamarind seed coat extract was measured in terms of hydrogen- donating or radical-scavenging ability, using a stable 2,2-diphenyl-1-picrylhydrazyl radical (DPPH Sigma-Aldrich Chemie, Steinheim, Germany). The assay is based on hydrogen atom- or electron-donating ability measured as bleaching of the purple-colored methanolic DPPH. In a 96-well plate, 75 μl of various concentrations of the extract (0.5–1000 μg/ml in methanol) was added, followed by 150 μl of 0.2 mM methanolic DPPH. After incubation for 30 min at room temperature, the absorbance was measured spectrophotometrically at 515 nm against a methanolic DPPH blank (without the test sample). L-ascorbic acid (POCH SA, Slaskie, Poland) and α-tocopherol (Sigma-Aldrich) were used as antioxidant standards. The radical-scavenging activity was calculated as a percentage of DPPH decol- oration using the following equation: %Free-radical scavenging = [1 – (A(sample)/A(blank))] × 100 where A(sample) is an absorbance intensity of sample solution and A(blank) an absorbance intensity of blank solution. EC50, the equivalent concentration to give the 50% effect, was determined by log-probit analysis using 10 different fi nal concentrations of the sam- ples. The study was performed in triplicate. CYTOTOXICITY OF THE EXTRACT TO HUMAN SKIN FIBROBLASTS Cell isolation and cultivation. Fibroblasts were obtained from the eyelid of a woman, aged 65 years, after routine plastic surgery. The procedure was approved by the ethical com- mittee of Naresuan University. The dermis layer of human excess surgery skin tissue was cut into small pieces by a surgical blade. Four to fi ve skin disks were then placed in a culture dish and subsequently incubated at 37°C with a humid atmosphere containing 5% CO2 for 30 min. The culture medium consisted of Dulbecco’s modifi ed Eagle’s medium (DMEM low glucose Sigma-Aldrich) and 10% fetal bovine serum (FBS Cultilab, Campinas, São Paulo, Brazil), and 1% stock penicillin/streptomycin (GIBCO/Invitrogen Corporation, Grand Island, NY) was added to each fl ask. After incubation, the fi broblast
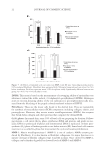
JOURNAL OF COSMETIC SCIENCE 14 cells migrated from the original attachment site. The cells were cultured in DMEM/FBS in air/5% CO2 and temperature of 37oC. Cell treatment. To determine the cytotoxicity of the extract, we transferred the cell suspen- sion (not exceeding eight passages) from a 175-cm2 fl ask into a 96-well plate (1 × 104 cells per well). After 24 h incubation, cells were exposed to 50–200 μg/ml extract for 24 h. The control cells were cultured in DMEM without extract. The viability of cells was determined by 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]- 2H-tetrazolium hydroxide (XTT Boehringer Mannheim, Mannheim, Germany) assay (17,18). Briefl y, following incubation, the old medium was replaced with 200 μl serum- free DMEM and 50 μl XTT-labeling mixture was added. The samples were further incu- bated for 4 h. The intensity of dye was measured with a spectrophotometer at 490 nm. The fraction of viable cells was calculated by subtracting the optical density fraction of treated cells from the untreated cells. These determinations were performed in triplicate. EFFECTS OF THE EXTRACT TO H2O2-DAMAGED FIBROBLASTS A suspension of fi broblasts from the primary cultures (not exceeding eight passages) was transferred from a 175-cm2 fl ask into a 24-well plate (1 × 105 cells per well) and then incubated at 37°C under 5% CO2 for 24 h. After treatment with 200 μg/ml tamarind seed coat extract, cells were treated with various concentrations (100–1000 μM) of H2O2 to induce oxidative stress in fi broblast (19). The control cells were not exposed to H2O2 or the extract. The viability of cells was determined by XTT assay. The cytotoxicity of H2O2 on cells was quantifi ed and expressed as percentage of cell damage relative to 0% damage calculated from the number of control cells. The study was performed in tripli- cate. DETERMINATION OF PREVENTIVE EFFECTS OF THE EXTRACT TO UVA-INDUCED CELL ALTERATIONS UVA irradiation. The UVA source was generated by a Honle F-lamp with a H-1 band- pass fi lter (320–400 nm) and the output intensity measured by a meter with UVA probe (Honle, Gräfelfi ng, Germany). A suspension of fi broblasts from the primary cultures (not exceeding eight passages) was transferred from a 175-cm2 fl ask into 12-well plates (2 × 105 cells per well). There were three treatment groups: (1) no extract, no UVA irradiation (control group) (2) no extract plus UVA irradiation and (3) extract pretreatment plus UVA irradiation group. For group 3 cells, tamarind seed coat extract was added directly to the medium (200 μg/ml) and all the groups were similarly incubated for 24 h (37°C/5% CO2) after which they were 80–90% confl uent. To avoid UV being absorbed or creating adjuncts, all media were replaced by phosphate-buffered saline (PBS without extract). Some of the plates (groups 2 and 3) were UVA irradiated (40 J/cm2) after which the PBS in all the plates was replaced by culture media. After this, the cells of each group were studied as follows: Cell viability: After 24 h of irradiation, the XTT assay was used to assess cell viability. The optical density of the control group was adjusted to 100%.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)