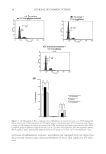
MOLECULAR MARKER APPROACHES FOR TRACKING REDOX DAMAGE 29 An ESI-MS/MS spectrum showing tyrosine photooxidation in the keratin-derived pep- tide with native sequence SFGYR is shown in Figure 2. This peptide met the criteria for use as a molecular marker of redox damage. Using MS/MS fragmentation data, a range of photo-induced redox modifications were thus characterized and located within wool keratins. Those peptides meeting the criteria for potential utilization as redox damage markers are listed in Table I, along with observed UVA-induced photomodifi cations. DAMAGE TRACKING To validate redox damage tracking for keratins, the relative abundance of selected oxida- tive modifi cations to keratin marker peptides was evaluated over differing irradiation protocols. Parallel quantitative evaluation of both the ion intensity and the ion peak area of each oxidative damage product was performed relative to the unmodifi ed native pep- tide within each sample, with each sample analyzed in triplicate. The two differing quan- titative methods were employed in parallel both for comparison purposes and to demonstrate the validity of the marker peptide approach for both methods. This is sum- marized for two peptides identifi ed as good candidates for use as molecular redox damage markers in Tables II and III. In the keratin marker peptide SFGYR, blue light produced an initial increase in the relative abundance of the tyrosine primary oxidation products, dopa, likely due to the UV component of the blue light utilized (11), but this was subsequently photo- bleached by further irradiation, consistent with the absorption of blue light (i.e., Figure 2. ESI-MS/MS spectrum of the keratin-derived peptide SFGY*R (m/z 645.3), where tyrosine (Y) has been photomodifi ed by UVA exposure to dihydroxyphenylalanine (dopa) (Y*), with selected fragment ions highlighted.
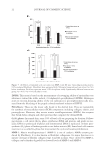
JOURNAL OF COSMETIC SCIENCE 30 dopa acting as a yellow chromophore) leading to further modifi cation. For UVA and UVB irradiation, the level of the oxidized marker peptide increased with irradiation time, with the native marker peptide being completely destroyed after 72 h of UVB irradiation. In the keratin marker peptide, LASDDFR, the damaged peptide in which the phenylalanine residue was oxidized to tyrosine was found to increase steadily both during UVA and UVB, and blue light irradiation, consistent with the initial photoproduct, tyrosine, absorbing only UV light, and therefore not photobleaching in blue light. The photobleaching effect of blue light on protein-bound chromophores formed through UV exposure, of particular relevance to the indoor light stability of carpet Table I Summary of Selected Keratin Damage Marker Peptides and Their Observed UVA-induced Photomodifi cations. Modifi ed Residues Are Marked in Bold Photomodifi cation Photoproduct Marker peptide Phenylalanine Hydroxylation [+O] Tyrosine LQFFQNR LASDDFR Dihydroxylation [+2O] Dopa LQFFQNR FAAFIDKEIR Tyrosine Hydroxylation [+O] Dopa LASYLEK SFGYR Dihydroxylation [+2O] Topa SFGYR Tryptophan Hydroxylation [+O] Hydroxytryptophan DVEEWYIR Oxidative ring opening [+2O] N-Formylkynurenine DVEEWYIR Oxidative ring opening [+2O], deformylation [-CO] Kynurenine DVEEWYIR Table II Dopa Formation From Tyrosine in the Keratin Wool Peptide, SFGYR (Unmodifi ed Tyrosine m/z 629.3, Modifi ed Dopa m/z 645.3) After Blue Light, UVA or UVB Irradiation, Expressed as the Abundance of the Modifi ed Peptide Relative to the Unmodifi ed Peptide, as Assessed Using Ion Peak Intensity or Peak Area Peak intensity Peak area Treatment m/z 629.3 m/z 645.3 Y*:Y m/z 629.3 m/z 645.3 Y*:Y Blue Light 3 h 11,114 464 4.2 756.467 51.526 6.8 24 h 1,668 407 24.4 145.956 46.665 32.0 72 h 3,363 174 5.2 225.219 20.098 8.9 UVA 3 h 9,685 1,168 12.1 755.166 150.045 19.8 24 h 11,256 2,537 22.5 1,000.694 330.6521 33.0 72 h 908 435 47.9 82.756 40.423 48.8 UVB 3 h 1,659 273 16.5 144.962 25.876 17.9 24 h 434 551 127.0 55.983 63.704 113.3 72 h N/A N/A N/A N/A Sequence: SFGYR Location: K2M2 res 62-66l Modifi cation: Y→[Y+O]. Y* is the modifi ed residue.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)