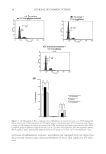
ANALYSIS OF DISTRIBUTION OF WATER IN HAIR 43 One observation in this study is that the scattering intensity corresponding to q = 0.07 and 0.13 Å−1 did not change signifi cantly with the amount of water (D2O) in the sample after the fi rst hydration up to 50 %RH. This suggests that the fraction of water in the vicinity of the IFs in the cortical cells remains relatively constant over the 50–95% humidity range. This was also refl ected in the increase in the d-spacings. The increase in the d-spacings cor- responds to a swelling of ~13%. However, Fig. 3 shows that the amount of water absorbed reaches 12% at 50 %RH, and then accelerates to reach value of ~25% as the humidity is increased to 95%. As amount of absorbed water increases with humidity, it shows that the additional water is present in regions other than the IF assemblies. The amount of water in these regions is expected to be different from that bound to the amide groups in the protein matrix. These assertions are supported by depolarization thermal-current (DTC) study in which ice-like water was reported at 19% water content (15). This water does not have the rotational properties of free water but has the polarization properties of the water molecules in the liquid state. Note that the 19% water content at which this new water peak appears in the DTC study is about the same value at which the amount of water accelerates in sorp- tion isotherm experiments (Fig. 2). The central diffuse scattering (CDS) observed near the beam stop suggests that water could be present in mesoporous (void structures between 100 and 1000 nm in size) regions in hair. Preliminary results in fact show a decrease in this in- tensity in hair exposed to D2O, perhaps because of contrast matching. More than one struc- tural feature contributes to the CDS (16). Important among these are the refraction from the macroscopic surfaces (hair) and microscopic surfaces (internal structure) (10), microvoids within the hair, and fi brils. Because the surface scattering and refraction from surfaces ap- pear in the form of elongated streaks (10), absence of such elongated streaks along the equa- tor allows us to attribute the CDS to internal structures and microvoids within hair. Although water sorption data can be used to determine the pore volume distribution in hair, it will not be accurate, because water swells the hair as it is absorbed, thus continu- ally changing the pore size. Mercury intrusion porosimetry is commonly used for porosity determination. In this method, mercury is forced into the void structure of the fi ber sub- strate under very high pressures (17). This method is generally not used for organic fi bers because high pressures used deform the pore structure in the fi ber. The standard method for determining the pore size distribution in solid substrates such as fi bers is nitrogen adsorption at low temperatures, using Kelvin equation to convert the sorption data into pore volume (18). Gas adsorption measurements indeed show that pores are present in hair, and their distribution changes during various hair treatments (19). The presence of pores is confi rmed by recent detailed electron microscopy studies (20), atomic force mi- croscopy studies (21), and optical measurements (22). Pores in the medullary features form a small fraction of the bulk of hair. Thus, water present in the keratinous structure gives rise to the interference peaks, and water present elsewhere, e.g., longitudinal pores, perhaps in the cortex and the cuticle, contributes to the CDS. SANS OF CUTICLE-FREE HAIR To determine if cuticular sheath is the locus of larger fraction of water in hair, we repeated the scattering experiments with CF hair. Samples of CF hair were prepared in the same way as regular hair as described earlier. Moisture-free samples of CF and regular EDBH were fi rst prepared by keeping them under vacuum in the presence of phosphorous pent- oxide. SANS patterns of the dry (no H2O or D2O) CF-free hair are shown in Fig. 5A.
JOURNAL OF COSMETIC SCIENCE 44 These patterns are similar to those of wet (D2O) samples in Fig. 4 with a typical dia- mond-shaped halo around the beam stop and a faint dumbbell. The SANS images from these samples appear different because the data were collected under different instrument confi gurations. However, they provide the same information as the patterns in Fig. 4. SANS patterns from CF hair that were fi rst placed in vacuum to remove water and then equilibrated with vapor of D2O at 6, 50, and 95 %RHDs are shown in Figs. 5B–D. The intensity is enhanced at 50 and 95 %RHD because of the presence of D2O, indicating signifi cant amounts of water diffusing into the matrix. The scattered intensity in both the as-received and the CF hair are quite similar. This suggests that cuticle sheath does not hold any signifi cant amount of water, and that most of the water is present in the medul- lary and the microfi bril regions of the hair. ABSORPTION OF OIL IN HAIR Earlier studies have shown a greater permeation of saturated vegetable oils such as coco- nut oil into hair than the polyunsaturated oils such as soybean oil (23). Heat treatment Figure 5. 2D SANS images of cuticle-free hair. The dark spot is the shadow of beam stop that blocks the main beam. Side bar shows the intensity scale. (A) Dry hair, (B) 6 %RHD, (C) 50 %RHD, and (D) 95 %RHD.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)