EFFECTS OF TAMARIND SEED COAT EXTRACT ON HUMAN SKIN 15 Cell-cycle analysis: After 72 h of irradiation, the proportions of cells at cell-cycle stages G1, S, and G2 were determined by the DNA stain, propidium iodide (PI Sigma-Aldrich). Cells were detached by trypsinization and washed twice with PBS containing 2 mM EDTA (Sigma-Aldrich). The cells were fi xed overnight with absolute ethanol (−20°C) and then stained with a solution containing 10 μg/ml PI, 10 μg/ml RNAse (GIBCO/ Invitrogen Corporation), and 2 mM EDTA in PBS. After 20 min incubation at room temperature in the dark, fl uorescent cells were sorted in a fl ow cytometry system equipped with a 488-nm argon laser (model FACScalibur, Becton Dickinson, Franklin Lakes, NJ). Cell-cycle data were analyzed using CellQuest Pro software. Total glutathione content: After 6, 24, 48, and 72 h of irradiation, cells were collected and lysed with T-PER lysis buffer (Pierce Biotechnology, Rockford, IL) for 20 min, and the resultant samples were centrifuged (5000g/5 min) to collect the supernatant. Total glu- tathione (GSH/GSSG) used was a commercial kit (Dojindo Molecular Technology, Ku- mamoto, Japan). To each well of 96-well plates, 20 μl enzyme solution, 140 μl coenzyme solution, and 20 μl of either GSH standard solution or the test supernatant were incu- bated at 37oC for 10 min. 5-5’-Dithio-bis(2-nitrobenzoic acid) solution (20 μl) was then added, and the samples were further incubated at 37oC for 20–40 min. Absorbance was measure at 415 nm on a microplate reader (Spectra Count PerkinElmer, Waltham, MA), and GSH content read off a calibration curve. MMP-1 and type I procollagen content: An enzyme-linked immunosorbent assay was used to quantitate human MMP-1, pro- and active forms, and newly synthesized type I procol- lagen in fi broblast cell culture supernatants. After 24–72 h of irradiation, cell-free super- natants were collected and stored at −80°C until used. The amount of MMP-1 and type I procollagen was measure by using a commercial human MMP-1 EIA kit (RayBiotech, Norcross, Germany) and a commercial human procollagen type I C-peptide EIA kit (Ta- kara Bio, Shiga, Japan), respectively. The levels of MMP-1 and type I procollagen were normalized against a standard dose–response curve based on the absorption at 450 nm using a microplate reader. The experiments were performed in triplicate. STATISTICAL ANALYSIS All quantitative data were expressed as means of samples for each treatment. Student’s unpaired t-test was used for comparison between two groups. p 0.05 was considered signifi cant. RESULTS AND DISCUSSION CHARACTERISTICS OF THE TAMARIND SEED COAT EXTRACT AND TOTAL PHENOLIC CONTENT The crude extract from tamarind seed coat was light-brown powder. The percent yield of the extract obtained was 22.0 ± 0.8% w/w. As determined by Folin–Ciocalteu assay, the amount of the phenolic compounds was 58.0 ± 0.3 g gallic acid equivalents per 100 g extract.
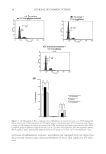
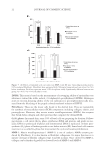
JOURNAL OF COSMETIC SCIENCE 16 FREE RADICAL SCAVENGING ACTIVITY OF THE EXTRACT Several assays including DPPH, 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid), and ferric reducing antioxidant power have been used to measure antioxidant capacities in plant extract (20–22). Most assays are based on the ability of the sample to scavenge a synthetic colored radical or to reduce the redox-active compounds. Among them, the DPPH oxidative assay is convenient in its application and thus is used worldwide in the quantifi cation of radical-scavenging capacity (23,24). In this study, the antioxidant activity of the extract was measured in terms of radical- scavenging ability, using DPPH. Such activity is inversely proportional to EC50 value, which was calculated from the nonlinear regression of the percentage of radical-scavenging activity against the sample concentration (Fig. 1). The lower EC50 value indicates the higher antioxidant activity. According to the obtained results, the EC50 value of tamarind seed coat extract was 12.9 μg/ml whereas those of L-ascorbic acid and α-tocopherol were 22.9 and 29.3 μg/ml, respectively. This result accords with previous studies (13,25) in- dicating strong antioxidant activity of tamarind seed coat extract, probably residing in its phenolic compounds (14,26). TOXICITY OF THE EXTRACT TOWARD HUMAN SKIN FIBROBLASTS To determine the cytotoxicity of tamarind seed coat extract, fi broblast viability was tested at various extract concentrations (50–200 μg/ml) for 24 h. Viability was better than 90% (Fig. 2). Moreover, even at highest concentration tested (200 μg/ml), changes in fi bro- blast appearance could not be detected (data not shown). Thus, the extract at the concen- tration of 200 μg/ml was used for further studies. EXTRACT REDUCES H2O2-INDUCED CELL DAMAGE H2O2 is an ROS that readily diffuses into cells and is far more stable than superoxide and hydroxyl radicals. H2O2 can be converted into highly reactive hydroxyl radical in the presence of reduced transition metal such as ferrous or cuprous ions (27). As an oxidant, H2O2 damaged primary human skin fi broblasts in a concentration-dependent manner (62–92%, 100–1000 μM) (Fig. 3). The tamarind seed coat extract could reduce the Figure 1. Free radical scavenging activity of tamarind seed coat extract, L-ascorbic acid, and α-tocopherol.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)