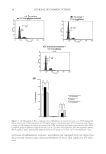
ANALYSIS OF DISTRIBUTION OF WATER IN HAIR 45 at high temperature often promotes the penetration of the oil deeper into the fi ber. To understand the mechanism of oil diffusion into the hair’s hierarchical structure, we pre- pared four tresses as mentioned in the experimental section. The SANS patterns of these tresses are shown in Fig. 6. The interference peaks are more intense in the heat-treated hair in both the coconut and the soybean oil-treated hairs, suggesting that more water present in the spaces between the IFs. This shows that heat treatment opens up addi- tional pathways, perhaps through the formation of new pores, for water to diffuse into the cortical regions of the hair. Detailed analysis (to be published) also showed that the interference peaks, both in the sample kept at room temperature and in the heat-treated sample, were stronger in the soybean oil-saturated hair than in the coconut oil-treated hair under the same conditions. This suggests that this diffusion-blocking effect is weaker with soybean oil than with coconut oil. This could be because of smaller amount of soybean oil penetrates into the fi ber and thus blocks fewer pathways for penetration of water than coconut oil (24). These results show that SANS is a useful technique for studying substrates that have been rendered hydrophobic by oil or other treatments, which block diffusion of water. Figure 6. 2D SANS images of hair treated with oil. The dark spot is the shadow of beam stop that blocks the main beam. Side bar shows the intensity scale. (A) Coconut oil—before heat treatment, (B) coconut oil—after heat treatment, (C) soybean oil—before heat treatment, and (D) soybean oil—after heat treatment. All at 90 %RHD.
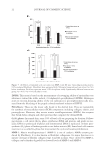
JOURNAL OF COSMETIC SCIENCE 46 HÙD EXCHANGE EFFECT OF D2O DIFFUSION INTO HAIR Polymeric substrates capable of hydrogen bonding with water when treated with D2O undergo HÙD exchange. Upon drying, this leaves some residual D in the sample. In hair, HÙD exchange can occur at pendant –NH2 (as in amino acid lysine as well as – CONH– functionalities), as shown by the following two schemes. The possibility of such exchange can be studied by infrared spectroscopy [Fourier trans- form infrared spectroscopy (FTIR) mode]. As the reduced mass increases when HÙD exchange occurs at N, both stretching and bending frequencies will be reduced signifi - cantly. For example, N–H frequency of the amine group (3219 cm−1) will be reduced to 2420 cm−1. But this frequency could not be followed in our experiments as it overlaps with that of CO2, and thus can be observed only with an FTIR spectrometer that can be fl ushed with nitrogen. Instead, we followed the bending frequencies of the amide func- tionality (amides I and II) (1632 and 1516 cm−1, respectively). FTIR spectra of HÙD exchange in hair are shown in Fig. 7A. The fi gure shows that the amide II peak shifts down with the substitution of H with D as expected. Treating this sample with water at 55°C should reverse this shift however, this was not observed although Murthy et al. (7) reported such a shift at this temperature following the deuterated amine end groups in nylon. In the present work, this reversal was observed only after treating the sample with boiling water (100°C). This can be seen in Fig. 7B that shows the upshift of the amide II peak following the substitution of D with H. CONCLUSIONS This preliminary SANS study of human hair has shown that water is present in essentially two forms: one that is adsorbed through hydrogen bonding and exchanged with amine and amide nitrogen in the spaces between the IFs and protofi laments, and the other in mesoporous re- gions as multimolecular layers and free D2O. Analysis of the interference peak due to water in the fi lamentary regions gives the spacing between the IFs to be 95 Å. A weak second-order
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)