MOLECULAR MARKER APPROACHES FOR TRACKING REDOX DAMAGE 27 HCl. The solution was kept stirring for 12 h (8). Wool fabric samples (6 cm × 6 cm) were treated with this silicon matrix solgel preparation by dip-coating for 5 min. The coated fabrics were allowed to dry overnight at room temperature and were annealed for 1 h at 120°C. IRRADIATION PROTOCOLS Keratin irradiation. Solid freeze-dried material was dissolved in water at 2 mg/ml and placed in ultraviolet (UV)-transparent quartz test tubes. The keratin solutions were irradiated with UVA, UVB, and/or blue light (9–11) for a period of 3, 24, or 72 h in an LZC4-14 photoreactor (Luzchem Research Inc., Gloucester, Ontario, Canada), at doses of 70,024 mWm−2 (blue), 52,670 mWm−2 (UVA), and 35,337 mWm−2 (UVB). Whole wool irradiation. Squares of untreated Merino wool fabric (3 cm × 3 cm) were treated with the solgel preparation and exposed to UVA and UVB irradiation (9–11) for varying periods to study the photoprotective effi ciency of the matrix treatments. All experiments were run in triplicate. Color evaluation. Squares of wool fabric treated with various silicon matrix preparations were exposed to UVA irradiation. Triplicate color measurements before and after UV ir- radiation were taken using a Minolta Chroma Meter CR210 (Osaka, Japan), with a wide area illumination, a 0° viewing angle, and a 50-mm diameter measuring area to average the reading over a wide area, as suitable for measuring cloth or textured surfaces. This yielded Y-Z values in CIE color space, which correspond very closely to yellowness as perceived by the human eye. A large Y-Z value indicates a very yellow sample. Digestion. After irradiation, the wool was digested using an in-house established protocol: solubilization buffer (180 μl 8 M urea, 50 mM Tris, 50 mM tris(2-carboxyethyl)phos- phine, 2 M thiourea, pH 8) was added to 6 mg of the wool and vigorously stirred over- night at 30°C. The extract was then alkylated with 360 mM acrylamide and the proteins from a 100 μl subsample were precipitated with 400 μl methanol, followed by 100 μl chloroform and then 300 μl water. The precipitate thus obtained was washed with 400 μl methanol, centrifuged at 14,100 g, and the methanol layer removed without disturbing the pellet. The pellet was suspended in 0.1 M ammonium bicarbonate–dimethylformamide (ratio: 7:3) and digested overnight at 37°C with trypsin (1:50), dried in a vacuum centrifuge, and stored at 4°C until further use. The tryptic peptides generated were desalted prior to direct infusion mass spectrometry (MS) with Proxeon StageTips (Prox- eon, Denmark). Chromatography. High-performance liquid chromatography (HPLC) analyses of the kera- tin digests were performed on a Bio-Rad BioLogic DuoFlow system equipped with a QuadTech multiwavelength UV-Vis detector, using a reversed-phase Bio-Rad RP-318 300 Å wide pore C18 column. Mass Spectrometric Analysis: Electrospray Ionization Tandem Mass Spectrometry. Mass spectro- metric analysis was performed on a tandem quadrupole-time-of-fl ight mass spectrometer (QSTAR Pulsar i, AB SCIEX, Framingham, MA), utilizing direct infusion nanospray of aqueous samples. LC-MS/MS was carried out on an Ultimate nanofl ow HPLC equipped with Famos autosampler and Switchos column switching module (Dionex, Sunnyvale, CA). Subsamples (10 μl) were loaded on a C18 trap column (5 mm, 300 μm inner diameter) at
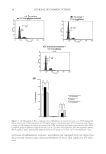
JOURNAL OF COSMETIC SCIENCE 28 a fl ow rate of 8 μl/min. The trap column was then switched in line with the analytical column (C18, 30 cm, 75 μm ID). Both trap column and analytical column were in-house packed with Varian Microsorb C18 5 μm, 300 Å particles. Reversed-phase gradients were from 2% to 55% solvent B at a fl ow rate of 150 nl/min over 50 min. Solvent A was 0.2% formic acid in HPLC-grade water solvent B was 0.2% formic acid in LCMS-grade aceto- nitrile. Loading and desalting solvent was 0.2% formic acid, 2% acetonitrile in HPLC- grade water. The analytical column outlet was directly connected to the mass spectrometer using a stainless steel nanospray needle (Proxeon). Triplicate technical repeats were per- formed for all samples. RESULTS AND DISCUSSION SELECTION OF MOLECULAR MARKERS To characterize redox modifi cation and select marker peptides for oxidative damage, a simplifi ed system of enriched wool proteins was fi rst evaluated. Type I and II keratins were isolated and purifi ed from whole wool utilizing reductive sulfi tolysis (12). Solutions of these keratins were irradiated with UVA for periods between 0 and 72 h. The photo- modifi ed proteins thus produced were enzymatically digested into peptide mixtures. Chromatographic analysis indicated substantial photomodifi cation of the wool-derived proteins with increasing irradiation times, as demonstrated in Figure 1 (peptide oxida- tion is expected to result in an increase in UV absorbance due to the formation of UV- absorbing chromophores (13,14). Electrospray ionization tandem mass spectrometry (ESI-MS/MS) was utilized to search for sequence-modifi ed keratin-derived peptides and to characterize modifi cations correspond- ing to redox-modifi ed residues. Certain residues, particularly the aromatic amino acid resi- dues, were expected to be sensitive to UVA and UVB irradiation through type I and type II photooxidative mechanisms (15–17). From this evaluation, marker peptides for damage tracking were selected. The criteria utilized for the selection of markers were as follows: • The peptide contained a photosensitive aromatic residue: phenylalanine, tyrosine, tryptophan, and/or histidine. • The peptide was reproducibly observed in ESI-MS analysis of the unfractionated keratin tryptic digests with a high relative ion intensity. • The peptide ion m/z and the m/z of corresponding major photoproduct ions were clear of interfering ions in the unfractionated analysis. Figure 1. HPLC traces of keratin digest solutions corresponding to UVA irradiation times of 0, 3, 24, and 72 h (monitored at 214 nm).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)