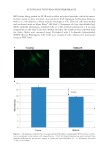
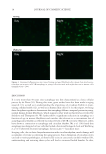
J. Cosmet. Sci., 67, 13–20 (January/February 2016) 13 Autophagy in human skin fi broblasts: Comparison between young and aged cells and evaluation of its cellular rhythm and response to Ultraviolet A radiation NADINE PERNODET, KELLY DONG, and EDWARD PELLE, Estee Lauder Research Laboratories, Melville, NY (N.P., K.D., E.P.), and Environmental Medicine, New York University School of Medicine, New York, NY (E.P.). Accepted for publication November 3, 2015. Synopsis Autophagic mechanisms play critical roles in cell maintenance. Damaged organelles that are not removed by autophagosomes, which act by engulfi ng and degrading these cellular components, have been linked to various pathologies. Recently, the progression of aging has also been correlated to a compromised autophagic response. Here, we report for the fi rst time a signifi cant reduction in autophagic levels in synchronized aged normal human skin fi broblasts as compared to young fi broblasts. We measured a 77.9% reduction in autophagy as determined by reverse transcription-polymerase chain reaction for LC3B expression, a microtubule- associated protein correlated to late stage autophagosome formation. In addition, we visualized these same changes by immunocytofl uorescence with antibodies directed against LC3B. By harvesting synchronized, as well as unsynchronized cells over time, we were also able to measure for the fi rst time a nighttime peak in autophagy that was present in young but absent in aged fi broblasts. Finally, since human skin is constantly subjected to environmentally induced oxidative stress from sunlight, we exposed fi broblasts to 10 J/cm2 ultraviolet A and found, in good agreement with current literature, not only that irradiation could partially reactivate autophagy in the aged cells, but also that this increase was phase shifted earlier from its endogenous temporal pattern because of its loss of synchronization with circadian rhythm. INTRODUCTION Autophagy, a major cellular degradative and recycling pathway, has now been shown to be essential for health and longevity, as well as a critical player in the aging process (1). As a highly conserved mechanism, it is responsible for the continuous recycling and renewal of intracellular organelles, lipids, and proteins and is a vital secondary source of energy for cells. It is a well-calibrated pathway that supports cellular homeostasis and responds to stress. By ensuring that malfunctioning or damaged intracellular components are removed and recycled, further intracellular damage can be minimized. The autophagic process removes cellular debris by fi rst sequestering material in an autophagosome, followed by Address all correspondence to Nadine Pernodet at npernode@estee.com.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)