JOURNAL OF COSMETIC SCIENCE 16 RNA ANALYSIS RNA was extracted with the RNeasy Kit (Qiagen, Valencia, CA) and prepared as directed in the manufacturer’s instructions. RNA concentration was determined by UV absorbance at 260 nm. For reverse transcription-polymerase chain reaction (RT-PCR), RNA was reverse transcribed using a High-Capacity cDNA Archive Kit (Applied Biosystems, ABI, Foster City, CA), as per the manufacturer’s instructions. Real-time PCR was employed using the 2X TaqMan Fast Universal PCR Master Mix (ABI) in conjunction with a 20X TaqMan Gene Expression Assay Mix, which consists of human primers and probes for MAP1LC3 (ABI, cat# Hs00797944_s1) and the endogenous control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ABI cat# Hs99999905_m1). Relative gene expression [change in expression of target gene normalized to an endogenous control (GAPDH) and relative to a reference group (untreated cells)] of real-time RT-PCR data was calculated using the 2-ΔΔCT method. Figure 2. Cell synchronization was induced by nutrient deprivation followed by nutrient repletion, which induced a temporal rhythm that showed an increase in autophagy by 77.9% (±10.9% SEM) in young fi bro- blasts, whereas this effect was absent in aged fi broblasts. LC3B expression was determined by RT-PCR and normalized to GAPDH housekeeping genes (n = 3).
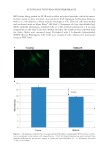
AUTOPHAGY IN HUMAN SKIN FIBROBLASTS 17 RESULTS Normal human dermal skin fi broblasts derived from either 2-day-old or 67-year-old donors were probed with antibodies directed against LC3B antigens for determining the level of autophagy. Immunocytofl uorescence revealed a marked decrease in autophagy in the mature fi broblasts as compared to the young fi broblasts [23.8% ± 1.9% standard error of mean (SEM)] and these data are shown in Figures 1A and B. When fi broblasts were synchro- nized by nutrient deprivation and then repleted with full media, signifi cant increases over time (77.9% ± 10.9% SEM) in LC3B transcripts as determined by RT-PCR were mea- sured after 2 h in the young fi broblasts, whereas in the aged fi broblasts this effect was absent (Figure 2). Interestingly, we also observed this relationship in the unsynchronized cells albeit to a much lesser degree. Under both conditions, we found a distinct biphasic temporal pattern, which correlated to the night in Zeitgeber time. As a control to ensure that our model system was respon- sive to changes in circadian patterns, the clock gene transcript for per2 was also evaluated by RT-PCR. The results from this experiment showed a temporal pattern that correlated with the evening onset of this clock gene (8) and are shown in Figure 3. Thus, an increase in autophagy in young cells occurs in the evening that is not evident in mature cells. These data were also supported by cellular fl uorescent images captured over time under the same conditions for LC3B as shown in Figure 4 and clearly demonstrate higher levels of autophagy for young fi broblasts at night. Since it had been previously reported that oxidative stress is a potent activator of autophagy (9) and that sunlight is a source of this type of stress, we then exposed fi bro- blasts to 10 J/cm2 UVA and determined autophagy over time as before. In young cells under synchronization conditions, LC3B expression levels after irradiation were found to be similar to their endogenous levels. However, in mature cells, LC3B expression could be induced by 27.2% (±5.3% SEM) and the phase of the induction of autophagy was shifted earlier by 1 h. As shown in Figure 4, these data demonstrate a potential for reac- tivation of autophagic mechanisms. Figure 3. Per2 expression that increases in the evening was assayed by RT-PCR over time in parallel to the synchronization experiments in young fi broblasts to confi rm that evening Zeitgeber time in our experiments peaked at approximately 2 h after nutrient repletion (n = 3). Expression normalized to GAPDH housekeep- ing genes.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)