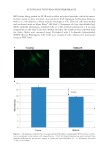
JOURNAL OF COSMETIC SCIENCE 18 DISCUSSION It is now more than 50 years since autophagy was fi rst characterized as a basic cellular process by de Duve (10). During this time, great strides have also been made in aging research (11), as well as in understanding the importance of circadian rhythm in main- taining cellular health (12), as well as in human skin cells (13). In this report, we bring these disciplines together to demonstrate that autophagy follows a temporal pattern in young, normal human dermal fi broblasts, which supports other work in this fi eld as reviewed by Sachdeva and Thompson (6). We further show a signifi cant reduction in autophagy as a function of age in mature fi broblasts and correlate this decrease to a concomitant loss of autophagosomal rhythm as refl ected by reduced levels of LC3B over time. Moreover, others have shown a connection to autophagy and circadian rhythm. Ma et al. (14) found that autophagy was disrupted in liver cells that lacked a functional biological clock and Weibking et al. (15) observed decreased autophagic factors in per1-/- knockout mice. In aging cells, due to their dissynchronization with circadian rhythm, much damage will accumulate over time accelerating the aging process. Since disruption of circadian cycles can lead to various pathologies, as observed by a rise in cancer rates in shift workers (16), understanding and restoring these cycles will lead to increased health benefi ts. In skin, Figure 4. Immunocytofl uorescence over time of young and aged fi broblasts after release from synchroniza- tion show an increase in LC3B/autophagy in young cells associated with night but not in mature cells (magnifi cation = 20×).
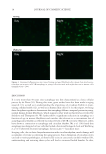
AUTOPHAGY IN HUMAN SKIN FIBROBLASTS 19 improvement in autophagy function may also lead to amelioration in the visible signs of cutaneous aging. Interestingly, after UV irradiation of cells, we also observed a slight reinduction of the autophagosomal response in those cells derived from mature donors. Thus, our data indicate the possibility of restoring autophagosomal function in aged skin. Since a phase shift in the timing of autophagy was also observed, resynchronization as a treatment modality should also be investigated. Future research should include investigation into the downstream effects of UV exposure in skin such as the generation of reactive oxygen species and how they may contribute to the activation of intracellular damage removal. In addition, oxidation-sensitive signaling cascades, such as mitogen-activated protein kinases (MAPK), which can lead to genetic activation of antioxidant response elements, may also be a productive area for future stud- ies and the utilization of fi broblasts from more donors should strengthen our conclusions. In conclusion, we report for the fi rst time that autophagy levels peak at night in young fi broblasts, whereas in aged human skin fi broblasts, there is a reduction in autophagy. We further show the effect that environmental stress has on the expression of autophagic processes, and demonstrate how autophagy may be potentiated under these condi- tions, which emphasize its importance in maintaining healthy skin. Figure 5. UVA irradiation of fi broblasts did not signifi cantly affect LC3B/autophagy levels in young cells. However, UVA signifi cantly increased LC3B/autophagy in mature cells by 27.2% (±5.3% SEM) and phase shifted its peak expression to 1 h.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)