JOURNAL OF COSMETIC SCIENCE 20 REFERENCES (1) D. C. Rubinsztein, G. Marino, and G. Kroemer, Autophagy and aging, Cell, 146, 682–695 (2011). (2) T. Yorimitsu and D. J. Klionsky, Autophagy: Molecular machinery for self-eating, Cell Death Differ., 12 (Suppl 2), 1542–1552 (2005). (3) C. Zhan g and A. M. Cuervo, Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function, Nature Med., 14, 959–965 (2008). (4) A. M. Cuervo, Autophagy and aging: Keeping that old broom working. Trends in Genet., 24, 604–612 (2008). (5) F. Madeo, N. Tavernarakis, and G. Kroemer, Can autophagy promote longevity?, Nature Cell Biol., 12, 842–846 (2010). (6) U. M. Sachdeva and C. B. Thompson, Diurnal rhythms of autophagy: Implications for cell biology and human disease, Autophagy, 4, 581–589 (2008). (7) I. Tanida, T. Ueno, and E. Kominami, LC3 conjugation system in mammalian autophagy, Intl. J. Biochem. Cell Biol., 36, 2503–2518 (2004). (8) K. Yagita, F. Tamanini, G. T. J. van der Horst, and H. Okamura, Molecular mechanisms of the bio- logical clock in cultured fi broblasts, Science, 292, 278–281 (2001). (9) L. Lin, G. Ishdorj, and S. B. Gibson, Reactive oxygen species regulation of autophagy in cancer: Impli- cations for cancer treatment, Free Rad. Biol. Med., 53, 1399–1410 (2012). (10) P. Codogno, Shining light on autophagy, Nat. Rev. Mol. Cell Biol., 15, 153 (2014). (11) C. Lop ez-Otin, M. A. Blasco, L. Partridge, M. Serrano, and G. Kroemer, The hallmarks of aging, Cell, 153, 1194–1217 (2013). (12) T. Hunt, and P. Sassone-Corsi, Riding tandem: Circadian clocks and the cell cycle, Cell, 129, 461–464 (2007). (13) N. Pernodet and E. Pelle, “Chronobiology of the Skin,” in Harry’s Cosmetology, M. Rosen. Ed. 9th Ed. (Chemical Publishing Co., Revere, MA, 2015), Vol. 2, pp. 1241–1254. (14) D. Ma, S. Panda, and J. D. Lin, Temporal orchestration of circadian autophagy rhythm by C/EBPβ, EMBO J., 30, 4642–4651 (2011). (15) N. Weibking, E. Maronde, and A. Rami, Increased neuronal injury in clock gene per-1 defi cient-mice after cerebral ischemia, Curr. Neurovasc. Res., 10, 112–125 (2013). (16) E. S. Schernhammer, F. Laden, F. E. Speizer, W. C. Willett, D. J. Hunter, I. Kawachi, and G. A. Colditz, Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study, J. Natl. Cancer Inst., 93, 1563–1568 (2001).
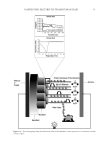
J. Cosmet. Sci., 67, 21–36 (January/February 2016) 21 Harvesting electricity from human hair BRINDAN TULACHAN, SUSHIL K. SINGH, DEEPU PHILIP, and MAINAK DAS, Biological Sciences & Bioengineering, Indian Institute of Technology Kanpur, Kanpur 208016, UP, India (B.T.) Solid State Physics Laboratory, DRDO, Delhi 110054, India (S.K.S.) Industrial & Management Engineering, Indian Institute of Technology Kanpur, Kanpur 208016, UP, India (D.P.) and Design Program, Indian Institute of Technology Kanpur, Kanpur 208016, UP, India (D.P., M.D.). Accepted for publication November 3, 2105. Synopsis Electrical conductivity of human hair is a debatable issue among hair experts and scientists. There are unsub- stantiated claims that hair conducts electricity. However, hair experts provided ample evidence that hair is an insulator. Although wet hair exhibited drastic reduction in resistivity scientists regarded hair as a proton semiconductor at the best. Here, we demonstrate that hair fi laments generate electricity on absorbing water vapor between 50° and 80°C. This electricity can operate low power electronic systems. Essentially, we are exposing the hydrated hair polymer to a high temperature (50°–80°C). It has long been speculated that when certain biopolymers are simultaneously hydrated and exposed to high temperature, they exhibit signifi cant proton hopping at a specifi c temperature regime. This happens due to rapid movement of water molecules on the polymer surface. This lead us to speculate that the observed fl ow of current is partly ionic and partly due to “proton hopping” in the hydrated nano spaces of hair fi lament. Such proton hopping is exceptionally high when the hydrated hair polymer is exposed to a temperature between 50° and 80°C. Differential scanning calorimetry data further corroborated the results and indicated that indeed at this temperature range, there is an enormous movement of water molecules on the hair polymer surface. This enormously rapid movement of water molecules lead to the “making and breaking” of innumerable hydrogen bonds and thus resulting in hopping of the protons. What is challenging is “how to tap these hopping protons to obtain useful electric- ity?” We achieved this by placing a bundle of hair between two different electrodes having different electro negativities, and exposing it to water vapor (water + heat). The two different electrodes offered directionality to the hopping protons and the existing ions and thus resulting in the generation of useful current. Further, by continuously hydrating the polymer with water vapor, we prolonged the process. If this interesting aspect of polymer is exploited further and fi ne tuned, then it will open new avenues for development of sophisticated polymer-based systems, which could be used to harvest electricity from waste heat. INTRODUCTION Human hair is a hot topic of debate on its electrical conductivity properties (1–5). To better understand the electrical properties of hair, the architecture of human hair is to be carefully examined (6–8). Human hair is a self-assembly of concentric, cylindrical, and structural composites with each concentric cylinder having different compositions and associated functional properties. It resembles monofi laments under microscope, Address all correspondence to Mainak Das at mainakd@iitk.ac.in
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)