JOURNAL OF COSMETIC SCIENCE 22 with roof tiles like overlapping scales, till the tip of the fi ber. The outermost concentric cylinder called cuticle consists of plate-shaped cells, scales, which overlap both longitudinally and peripherally. Cuticle is mainly composed of structural and free lip- ids surrounding the dead cells with inner volume of the cells fi lled with keratin pro- tein. The middle layer is the bulk of the volume consisting of spindle-shaped dead cells fi lled with α-keratin and keratin-associated proteins. However, the innermost cylindri- cal yet discontinuous layer consists of cells fi lled with melanin pigments, which is known for its semiconductor properties (9–12) and ultraviolet absorption capabilities (Figure 1 A, B). The overall architecture of the hair resembles a GAU-8 Avenger gun barrel of late Nineteen seventies (1970s) in nanoscale, where the metallic barrel is replaced with “protein nano- tubes”. Each spindle-shaped dead cell in cortex consists of fi ve to eight macrofi brils. Individual macrofi bril comprises of 500–800 microfi brils, where each microfi bril is made up of seven to eight protofi laments. Each protofi lament is a self-assembly of four chain structures called tetramer of α-keratin and keratin-associated proteins (Figure 1 C). α-Keratin protein is the major component of human hair (including wool), horns, nails, hooves, and claws of mammals (5,7,8). Eminent hair experts have provided ample proof that α-keratin protein in hair is an insulator (4,5,13–15) It has been demonstrated that resistivity value of hair changes with water content. The wool–water system with 7% water content, exhibited a resistivity of 3 × 1012 ohm cm at room temperature whereas, at 25% of water con- tent, the resistivity decreased to 6 × 106 ohm cm at room temperature. It has been concluded that even at high moisture content, α-keratin is a poor conductor of electricity (4,5,13–20). The scientists considered hair as a proton semiconductor to explain the limited electrical conductivity of a keratin–water system, which is the same as that of ice, nylon–water, and cellulose–water system (4,5,14,21–26). This conduction mechanism depends on a con- tinuous hydrogen bond network that is formed between the water and keratin molecules. This network facilitates conduction by proton hopping, which explains why wet hair exhibited lower resistivity. Figure 1. Anatomy of hair. (A) Longitudinal section of a single hair fi ber. (B) Longitudinal section of the hair showing the detailed cellular architecture. Transverse cross-section showing the large cortical region. (C) Arrangement of α-keratin protein inside the cortical cells.
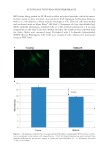
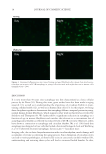
HARVESTING ELECTRICITY FROM HUMAN HAIR 23 In this report, we studied the electrical conductivity of dry hair, wet hair, and hair ex- posed to water vapor (water + heat) and performed a comparative study with two other natural fi brous proteins viz. mulberry (Bombyx mori) and non-mulberry Antheraea my- litta) silk (Figure 2)(26). Further using human hair, we developed a simple bio-electric device, which on exposure to water vapor generates suffi cient electricity so as to operate low power electronic systems. We have further performed a comparative differential scanning calorimetric (DSC) study of hair and silk fi ber in dry and hydrated conditions Figure 2. World of silk. (A) Schematic diagram showing the overall molecular architecture of silk cocoon and unprocessed silk thread. The silk cocoon is made up of these individual silk fi bers. Individual silk fi bers consists of two proteins viz. an outer gummy protein called sericin and an inner core forming the actual silk thread called fi broin. The silk threads adhere to each other by sticky sericin protein thus forming the rigid architecture of the silk cocoon. (B) A. mylitta silk cocoon, a wild non-mulberry silk. (C) Processed non- mulberry silk thread after degumming, which is used for textile. (D) Spider silk lacking gummy sericin protein and that is the reason why the individual threads could be observed. Figure 3. Conductivity and total dissolved solute (TDS) of the pure deionized water, deionized water after fi rst hair wash and deionized water after second hair wash. The hair was used after second wash for the experiment. A bath ratio of 5:1 of human hair in mg and deionized water in ml was used in each soak. Cyberscan Con 11 Eutech instruments was used at room temperature 25 °C for measuring the conductivity and TDS.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)