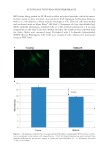
JOURNAL OF COSMETIC SCIENCE 24 and showed that a rapid motion of water molecules takes place at temperature around 80°C, which resulted in generation of large number of protons on the surface of these biopolymers. This partly explains the observed maximum conductivity is observed around 50°–80°C. This fi nding is important because if such devices could be developed using biopolymers, then these will be useful to harness electrical energy from waste heat contained in the exhaust steam of thermal and nuclear power plants, as well as Table I Hair and Silk Cocoon Membrane Confi guration I Confi guration II Confi guration III Electrode 1 (E1) Copper Platinum Copper Electrode 2 (E2) Copper Aluminum Aluminum Figure 4. Standard device fabricated by connecting the two similar and dissimilar electrodes to study the electrical properties of human hair and silk cocoon membrane. This uniform device helps in comparing the data obtained from different sources. (A) Two copper electrodes attached with colloidal silver paste for mak- ing proper connections. The fi rst device is prepared with human hair. Second and third devices were prepared with A. mylitta silk cocoon with average natural thickness 0.5 mm and B. mori silk cocoon membrane with an average thickness of 0.5 mm. (B) Simple device was made by connecting the two different electrodes on the surface composed of aluminum and platinum. (C) Device prepared with aluminum and copper electrodes.
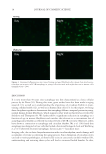
HARVESTING ELECTRICITY FROM HUMAN HAIR 25 from the buildings in the winter, where a large amount of heat energy is needed to maintain the temperature. MATERIALS AND METHODS COLLECTION OF HAIR SAMPLES AND PROCESSING Hairs from random individuals were collected, and washed with deionized water twice for an hour. This wet hair was then allowed to dry in a laminar fl ow hood at room tem- perature. One of challenging problem is to wash the hair sample and to ensure the re- moval of the ionic species (27). Thus we conducted a simple test to ensure that most of the loosely bound ionic species are removed during washing. We fi rst measured the conductivity of the deionized water to be used for washing the hair (Figure 3). Next, we kept the hair sample in this water and used a magnetic stirrer to swirl the water for 30 min, thus ensuring that all the loosely bound ionic species present in water gets dissolved in water. After the fi rst wash, we measured the conductivity of the water. We observed a signifi cant increase in conductivity, thus indicating the presence of ionic species which has leached out from the hair surface. Again we repeated the process for 30 min and measured the conductivity. We observed that the conductivity of water falls down to the baseline. This experiment ensured that the removal of the ionic spe- cies and proper washing of the hair. Figure 5. Hair bioelectric device. (A) All the components needed to fabricate a simple hair bioelectric de- vice. (B). The overall test apparatus showing the source of water vapor. (C) The bioelectric device inside the plastic casing attached to the glass tube supplying water vapor.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)