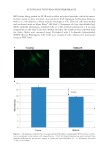
JOURNAL OF COSMETIC SCIENCE 26 COLLECTION OF MULBERRY (B. MORI) AND NON-MULBERRY (A. MYLITTA) SILK COCOONS The mulberry and non-mulberry silk cocoons were collected from the silk farmers resid- ing in the Indian states of Karnataka and Chattishgarh, respectively. These cocoons were cleaned with a dry blower and stored in a desiccator (26). SCANNING ELECTRON MICROSCOPY The morphology of the hair and silk cocoon membrane was studied using scanning elec- tron microscopy (SEM SUPRA 40VP fi eld emission SEM, Carl Zeiss NTS GmbH, Oberkochen, Germany). SELECTION OF ELECTRODE MATERIALS AND DEVELOPING THE MEASUREMENT SETUP Aluminum, platinum, and copper were chosen as the electrode metals. A glass slide was used as the holding frame, on which electrode (E1) was fi xed. Then, 150 grams of dried human hair was spread evenly covering the electrode (E1). In case of silk, the silk cocoon membrane was fi xed on top of E1. The counter electrode (E2) wire was wounded over the whole setup covering the hair/silk cocoon membrane substantially, forming the measurement device with hair/ silk sandwiched between the electrode Figure 6. Gross and scanning electron microscopic morphology of Human hair and Silk Cocoon. (A) Natu- ral human hair with black texture. (B, C) A. mylitta silk cocoon has brownish coarse texture while B. mori cocoon has a soft texture. (D) Scanning Electron Microscopy image of the surface of a human hair showing cuticle (scales) on outside of our hair. (E, F) Rough outer surface of A. mylitta with some crystals of calcium oxalates and smooth outer surface of the B. mori cocoon.
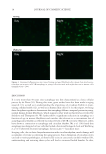
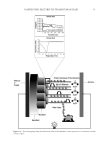
HARVESTING ELECTRICITY FROM HUMAN HAIR 27 metals. All voltage and current measurements were made using this “hair/silk mea- surement device”. Three different electrode confi gurations used in the study are shown in Table 1. Three different electrode confi gurations (I–III) were used for recording the current and voltages across hair and silk cocoon membrane. In confi guration I, same electrodes were used across the membrane. In confi guration II and III, different electrodes were used. For confi guration I, high-purity copper wires of 32 AWG (1.2-m long) and 32-gauge copper sheets (1 × 2 cm2 area) were used. While for confi guration II, high-purity platinum wire of 49 AWG (3-m long) and 32 gauge aluminum sheets (1 × 2 cm2 area) were used and for confi guration III, high-purity copper wires of 32 AWG (1.2-m long) and 32-gauge alu- minum sheets (1 × 2 cm2 area) were used as electrodes. We either use same copper electrode as E1 and E2 (Figure 4A), or dissimilar electrodes (Figure 4B, C). The fi gure 4A –C is showing some simple devices fabricated by us, using human hair and silk. Three different electrode confi gurations (E1:E2) were used to per- form the recordings. ELECTRICAL MEASUREMENTS The current and voltage across the measurement device was recorded using an electrom- eter Keithley’s 51∕2-digit Model 6517B electrometer/high resistance meter (Keithley Figure 7. Electrical properties of human hair and silk cocoon sandwiched between same copper electrodes (Device 1). Representative traces of current and voltage obtained from bioelectric device described in Figure 4. (A) Average (n = 6) current profi les of human hair under the two different conditions (moist and exposed to water vapor), in moist state the average current value is around 9.57185E-09 while it increases drastically to 8.65487E-06 when exposed to water vapor. (B, C) Trend is almost identical in the silk cocoon but the average current density is higher as compared to the human hair. When exposed to water vapor average current den- sity in (B) A. mylitta is 7.98428E-05 which is higher than (C) B. mori 6.48795E-05, as it has more ion species inherently present in them. (D) Average voltage recording obtained from human hair shows that there is negligible voltage when moist, while when exposed to water vapor the voltage rises sharply. (E, F) Pattern of average voltage reading of silk cocoon membrane is similar to that observed in human hair. Voltage is low in moist conditions and increases sharply, when exposed to water vapor.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)