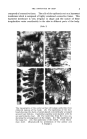
38 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS TABLE VI. Showing the fungistatic effect of single p-hydroxybenxoates and combinations in nutrient solutions at room temperature (The stereum purpureum used was supplied by the Forest Products Research Labora- tory, Princes Risborough, and all the other moulds by the Commonwealth Mycological Institute) (1) (2) (3) (4) (6) (7) (8) (10) In glucose broth 0'02 % methyl p-hydroxyben- zoate 0.02% ethyl p'-•tydro c•benz'•atc 0-02 % propyl p-hydroxyben- zoate 0.02% n butyi 35-hyd•xybe•'- zoate 0.02% isobutil •-hydroxyben- zoate 0'01% benzyl' •-hydroxyben- v-oate ß . 0'02 % Nipa-ester combination ' '64" 0'02 % Nipa-e•t•r combination "82121" 0'02% Nipa-e•t•r combination "82123" .. 0'05% phenol gillus n ige •' q- q- .4•p gill: gmst, da• + + + -- _ -- __ -- __ + Pent- Pe• cillium cilli• brevi- roq, caule f•r + + + + _ _ + + __ _ -- _ __ _ -- __ __ __ + + i- •m Mucor [e- rnucedo , . + + __ + -- -- -- __ + In malt water Stereum purpureum (1) 0.01% methyl p-hydroxybenzoate (2) 0.01% propyl .... (3) 0.01% Nipa ester combination •1 (4) 0.01% ...... 82121 (5) 0.01% 82123 (6) 0.01% ... '.'. .. {7) 0-01% phenol ...... (s) 0.0s% ........ practice, show that by increasing the number of powerful esters in a com- bination we have produced an outstandingly effective preservative, highly suited for use in the cosmetic and other industries for the preservation of fine quality products. CHEMICAL AND PHYSICAL PROPERTIES OF NIPA ESTERS AND NIPA ESTER COMBINATIONS The solubilities of the esters and combinations in an aqueous medium have been indirectly indicated in the foregoing tests. Their incorporation into cosmetic products, however, usually requires the use of an organic solvent or vehicle and Table VII shows the solubility values for a number of Nipa products in some useful solvents.
NIPA-ESTER COMBINATIONS AS PRESERVATIVES AND ANTISEPTICS 39 TABLE Vii. ]•ercentage $ol•bililies Solvent Methyl Ethyl Propyl n Butyl Nipa- Nipa Nipa Nipa ester ester ester ester sept 64 82121 82123 Water at 20 ø C. 0.25 0.07 0.032 0.0225 0.2 0-03 0.14 0'08 .... 25 ø C. 0.3 0.075 0.04 0-025 0-25 0.033 0.17 0-1 .... 80 ø C. 3-5 1'0 0-39 0-135 1.8 0.145 0.39 0.35 Methyl alcohol 35 45 50 70 55 90 65 65 Ethyl ,, 35 40 40 70 50 90 60 60 Acetone .. 35 40 50 60 50 90 60 60 Propylene glycol .. 20 15 20 40 20 90 35 35 Benzene 1 1 3 30 1 70 3 3 Carbon tetra' Less Less chloride .. than 0.1 than 0'1 0.1 5 0'1 30 0-4 0'4 Ether .. 10 15 25 30 20 80 35 40 Glycerol .. 1 0.5 0-4 0-4 2 0.4 5 1 Liquid paraffin Less Less Less Less Less Less Less than 0.1 than 0' 1 than 0-1 than 0.1 than 0-1 0.1 than 0.1 than 0.1 (Solubilities in all organic solvents are at 25 ø C. and are true percentages, i.e., by a 25 per cent value we mean 25 grams substance is soluble in 75 grams solvent.) OTHER CHEMICAL AND PHYSICAL PROPERTIES The various combinations of Nipa-esters described in this paper, viz., Nipasept, Nipa 64, Nipa 82121 and Nipa 82123, are white crystalline powders almost odourless, tasteless, but producing a slight burning sensation of the mouth and tongue, followed by local numbness. The limit tests for impurities such as chloride, sulphate, free acid and lead which are described in the British Pharmaceutical Codex 1954 for the methyl and propyl esters of p-hydroxybenzoic acid apply to these combinations. The combinations have the following melting ranges: Nipasept ...... 80-120 ø C. Nipa 64 ...... 50- 65 ø C. Nipa 82121 ... 60-120 ø C. Nipa 82123 ... 60-110 ø C. The combined p-hydroxybenzoic acid content of the combinations can be determined as follows: About 1 gram of the substance is accurately weighed into a conical flask. This is dissolved in 25 ml. of N sodium hydroxide solution and the resulting solution is gently boiled for thirty minutes. The water lost by evaporation is replaced and the flask is cooled down to room temperature. Titrate against N hydrochloric acid solution using 3 drops of bromo thymol blue as indicator. The end-point is indicated when the colour of the solution matches a buffer of pH 6.5 containing the same amount of indicator. The buffer solution is prepared by diluting a mixture of 25 mi. of M/5
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)