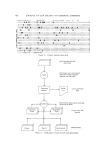
78O JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ,oo • •.,• I sf hour ""' - •' ,?_nd hour 8o '•L'. .............. .3rd hour 40 '\ '". 20 '• '"" I • 200 300 400 500 600 /• in rn)4 Fig. 1. Absorption spectra of trichosiderins in 0.1N HC1 with increasing length of extraction from hair. At arrow the concentration of the solutions was decreased to obtain readings at shorter wavelength upon neutralization small amounts of pigment may be precipitated from them. These late precipitates are increasingly brown their absorption bands at 535 m/• flatten and eventually disappear (Fig. 1), and their iron content steadily decreases (Table I). The organic portion of the pig- ment has no characteristic absorption bands and is complexed in acid solution through its combination with iron. Therefore it is likely that when the iron content drops below a certain level (about 0.1%), the pig- ment becomes insoluble and cannot be extracted anymore. Indirect evidence for this view comes from Dutcher and Rothman's iron determinations in hair of different colors (13). Black and blonde hairs were found to contain, on the average, 2.71 and 2.43 mg Fe/100 g, respectively red hair, on the average, 9.78 mg/100 g i.e., about 7 mg more per 100 g hair than the other varieties. Assuming an iron content of about 0.5% in the original pigment in situ, 7 mg iron corresponds to 1400 mg pigment. Actually, red hair yields 40-60 mg trichosiderin per 100 g of hair therefore only 3-5% of the total amount may be ex- tracted. As the iron content of the pigment in the hair itself is unknown,
THE COLOR OF RED IIAIR 781 'Fable I Iron Content of Hair and Feather Pigments after Varying Lengths of Extraction Time Length of extraction with boiling 0.1N HC1 (hr) Hair pigment (Fe r)•) Feather pigment (Fe %) 1 O. 38 O. 51 2 O. 24 O. 17 3 0.15 0.15 this calculation may be faulty.* Yet the basic observation is correct that the pigment is destroyed gradually during extraction. The authors believe that the iron pigment is a major, possibly the major, pigment of bright red human hair. In attempts to elucidate the chemical nature of trichosiderin, the re- search concentrated on the chromophore group which contains iron com- plexed with an organic moiety. The presence of iron in the molecule is responsible for its acid solubility, absorption band in the visible, indi- cator property, and neutral iso-electric point. The iron-free or iron-poor parts of the pigment have few characteristic features they are insoluble in acids, dissolve with a yellow-brown color in alkalies, and have no ab- sorption band in the visible. Trichosiderin proved to be a metallo-protein preliminary analyses indicate that it is composed soldy of iron, amino acids, and maybe some amines. } The protein nature is firmly established by a CHN ratio of 47.2-49.2, 6.8-7.3, 15.4-16.3 in all forms of the pigment, a positive Lowry reaction (14), typical infrared spectra, and amino acid analyses. Before carrying out extensive amino acid analyses, efforts were di- rected at obtaining a chromophore of the smallest possible molecular weight which still retains all the specific properties of trichosiderin. The method is essentially one of repeated acid extraction. The chromophore is soluble in acids and highly resistant to them at room temperature. Acid extraction of the chromophore with successive removal of the iron- poor parts is carried out at different levels: first with boiling 0.1N HC1 during the extraction of trichosiderin or feather siderin then with HC1 or 10% KSCN which splits off large amounts of brown, iron-poor material. The resulting chromophore becomes dialyzable in 0.iN HC1. * Dutcher and Rothman's data are based on an iron content of 10%, an unrealistic figure. At best, such a high iron content could be found in purified preparations only (5, 15) in the present work it was impossible to obtain such samples. } Improved analytical methods may account for the discrepancies between earlier reported data (5, 15) :tnd those reported herein.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)