J. Soc. Uosrnetic Uhemists, 20, 761-766 (Dec. 9, 1969) Photochemistry in Cosmetics NATHAN LEWIN, Ph.D.* Synopsis--Fate of electronic excitation energy is discussed in relation to photostability of cosmetic preparations and other detrimental light-induced phenomena. The principles and guidelines in search of suitable sunscreens and photostabilizers are discussed in the context of current theories. INTRODUCTION The instability to light of many cosmetic ingredients is well known and is evidenced by the vast number of products which require amber bottles or opaque containers. Recently, a great deal of interest has been focused on the phenomenon of photosensitization by various topical agents. The following is a list of some of the materials implicated in this manner (1-3). Dyes Sunscreen agents Perfumes Antihistamines Antibacterial agents Eosine, erythrosin p-Aminobenzoic acid esters and related materials Bergamot oil Promethazine Tetrachlorosalicylanilide, bithionol, sulfa drugs, coal tar derivatives, etc. Whether the phenomenon of "photosensitization," defined in this case as an adverse dermatological reaction caused by light, is photochemical in nature or whether it derives at least in part from immunological reac- tions is still a matter of conjecture. However, in view of this and other related problems, it is of interest to consider in greater depth some of the photophysical processes which precede the undesirable chemical reac- tions. * Consultant, P.O. Box 155, New York, N.Y. 10023. 761
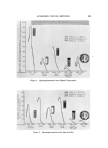
762 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS THEORETICAL CONSIDERATIONS Absorption of a photon of light results in raising of an electron from one of the low vibrational levels of the ground state, So, to one of the vi- brational levels of an excited singlet state, such as & or S2 (designated as process a on the Jablonski diagram, Fig. 1). Relaxation to a low vibra- tional level of the lowest excited singlet S• is usually very rapid (10 -•2 sec) and is shown as process b on the diagram. If the electron remains in S• for 10 -9sec or longer, the energy may be emitted as fluorescence (process c). Alternatively, the energy may be dissipated in a chemical reaction. Whereas in the singlet state the high energy electron retains its orig- inal spin, an inversion of this function may take place in a secondary step, referred to as intersystem crossing, to a triplet state T. This may take place directly to the lowest triplet, T•, shown on the diagram as process d, or by initial intersystem crossing to a higher triplet state, such as T2, followed by very rapid internal conversion to T• (radiationless process e). The lifetime of triplet states is usually much longer than that of the originally formed singlets and may be of the order of several seconds. Accordingly, many chemical reactions originate from triplet states. Other than via a chemical reaction, the triplet energy may be lost as heat or slow radiative emission, referred to as phosphorescence (process f). Direct absorption to a state such as T• or T• does not normally take place. However, T• can be generated directly by an interaction with another molecule which is in its triplet state, e.g., Tn, provided that ]'n ]'•. This phenomenon is known as sensitization. Singlet sensitiza- tion is also a common phenomenon in which the energy donor is in its singlet state. Conversely, loss of energy by one molecule to another is known as quenching (4, 5). • * T 1 RADIATIVE RADIAoeIONLESS Figure 1. Jablonski diagram
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)