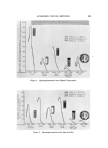
BUBBLES IN GELS 801 Figure S. o 0 IOO ALCOHOL-WATER MIXTURE / / O O O O 20 40 60 80 % ALCOHOL Results of alcohol-water mixing and Carbopol gel experiments ethanol content around 55%. It is possible, therefore, that the same mechanism may be responsible for air bubble release in both experiments. The volume of air in the Carbopol gels was calculated by taking the difference between the reciprocal of the density of aerated gel and that of bubble-free gel. Since, in the systems studied, the densities of the aerated gels were not greatly different from the densities of the cor- responding bubble-free gels, the results were very sensitive to small errors in measurements. For this reason, the data from the gel experiments were not as consistent as the data from the alcohol-water mixing experi- ments. Moreover, some loss of bubbles could not be completely pre- vented in the gel experiments during the mixing operation and equili- brating period. Although the extent of bubble loss is believed to be small, it could have contributed to the lower values of a in the gel experiments. Assuming that an identical bubble-formation mechanism is in opera- tion, another explanation for a lower a value in gel experiments may be formulated by considering surface tension. It is known that bubbles in a fluid are subjected to a pressure additional to the static pressure as the result of surface tension. This pressure is directly proportional to sur- face tension but inversely proportional to the diameter of the bubble (5). Therefore, the smaller the bubble, the larger will be the pressure inside the bubble. Many bubbles in the Carbopol gels were microscopic in dimensions and they were, therefore, subjected to this additional pres- sure which would make the total volume of bubbles smaller than the true volume at atmospheric pressure. This effect has no influence on the measurement in alcohol-water mixing experiments since the volume
802 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS was measured after all the undissolved air was collected in the buret. It is possible, therefore, that this effect was responsible for the smaller a value obtained in the gel experiments. It was reasoned that if the dissolved-air liberation mechanism due to mixing of the alcohol and water as illustrated in Fig. 1 were indeed re- sponsible for the bubble formation in the Carbopol gels, one should be able to prepare a nearly bubble-free gel by changing the manufacturing procedure. Since Carbopol resins do not gel until neutralized, any bub- ble formed prior to neutralization could escape from the mixture. Therefore, instead of neutralizing the Carbopol gel immediately after the water and alcohol are mixed, one could prepare gels by delaying neutrali- zation after the undissolved air in the alcohol-water mixture had a chance to escape. Theoretically, a Carbopol gel so prepared should contain no bubbles (although it may still contain some dissolved air) l•rovided that no external air is incorporated during the preparation. Similarly, .one should be able to prepare a bubble-free gel if all dissolved air were first removed by heat, vacuum, ultrasonic generator, or other means. To test the above theory, Carbopol gels containing 50% ethanol, 0.5% Carbopol 940, 0.5% triethanolamine, and 49• water were made using the following procedures: Procedure I (Control): Carbopol 940 was dispersed in ethanol, and triethanolamine was dissolved in water. The two solutions were mixed with a propeller mixer to form a gel instantly. This was the procedure used in the gel experiment. Procedure II (Delayed neutralization A): Carbot•ol resin was dispersed in a preblend of alcohol-ethanol. The dispersion was allowed to stand for several hours to free air bubbles before neutralization. Procedure III (Delayed neutralization B): The Carbopol resin was dispersed in alcohol, and water was added. The solution was mixed thoroughly and the mixture was allowed to stand for several hours to free air bubbles before neutralization. Procedure IV (Ultrasonic method): The Carbopol resin was dis- persed in alcohol, water was added, and then this mixture was passed through a laboratory ultrasonic homogenizer.* The homogenized mix- ture stood for 10 minutes to free cavitation bubbles before neutraliza- tion. As expected, the gel from Procedure I contained a considerable amount of air bubbles. The gels from Procedures II and III contained * Minisonic IV Ultrasonic Emulsifier, Sonic Engineering Corp., Norwalk, Conn.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)