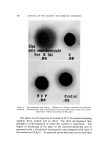
PHOTOCHEMISTRY IN COSMETICS 765 ciency of photoenolization must be considered in this context. For ex- ample, the transfer of triplet energy to one of the components of the preparation may compete with photoenolization and cause undesirable side reactions, particularly since the absorber is at low concentration compared to the substrate, which in this case is the "quencher." PHOTOSTABILIZATION BY QUENCHING Consideration of such energy processes or quenching of excited states leads to the discussion of some of the more recent approaches to the search for more effective and compatible photostabilizers. Theo- retical considerations (9) and recent experimental data indicate that the singlet energy can be transferred over a distance of several molecular diameters, i.d., up to 100A. On the other hand, the transfer of triplet energy takes place over much shorter distances and in the range of colli- sion diameters (10). In considering viscous and semisolid media such as are encountered in cosmetic preparations, the availability of a method of quenching singlets at a "distance," and therefore at low concentration of the additive, becomes attractive. This then is path B mentioned earlier in this discussion and represents a novel method of interfering with photodecomposition. Products in this category, e.g., chelates of nickel and other transition metals, are at present marketed on an ex- perimental basis although not as yet for cosmetic use (7). The quenching of triplets, method C, has not yet yielded photo- stabilizers. However, photochemical considerations lead to some very interesting conclusions as, for example, in substances VI (n = 0, 1, 2, 3) described by Leermakers and his coworkers (11) (Fig. 2). The light ab- sorbed by benzophenone gives rise to benzophenone singlet, SB, which --(C H.:,ln--•,• C • VI E •I TN -- S o -- Figure 2. Intramolecular energy dissipation
766 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS undergoes very rapid intersystem crossing to the corresponding triplet, TB. The latter is efficiently quenched by the naphthalene part of the molecule, even though the two parts of the molecule are not conjugated in the classical sense. TN then decays to ground state. On the other hand, light absorbed by the naphthalene part gives rise to naphthalene singlet, SN, which is efficiently quenched by the benzophenone to give benzophenone singlet, SB. The latter goes over to the triplet and the process is repeated. Products of this type which carry their own energy disposal systems may represent photostabilizers of the future. SUMMARY Mechanisms of photostabilization are discussed in terms of current photochemical theories. Energy transfer processes are reviewed in the light of potential use in cosmetic applications. (Received February 13, 1969) REFERENCES (1) Vinson, L. J., B orselli, V. F., and Singer, E. J., Realistic methods for determining photo- sensitization potential of topical agents, Am. Perfumer Cosmetics, 83, 37 (May, 1968). (2) Strobel, A., and Inserra, J. J., The use of U.V. absorbers in cosmetic products, Ibid., 83, 25 (December, 1968). (3) Rottier, P. B., Biologic problems concerning sunscreens, J. Soc. Cosmetic Chemists, 19, 85 (1968). (4) Calvert, J., and Pitts, J. N., Jr. Photochemistry, John Wiley & Sons, Inc., New York, N.Y., 1966. (5) Turro, N.J., Molecular Photochemistry, W. A. Benjamin, Inc., New York, N.Y., 1965. (6) Yang, N. C., Zwicker, E. F., and Grossweiner, L. I., The role of n-r* triplet in photo- chemical enolization of o-benzylbenzophenone, J. Am. Chem. Soc., 85, 2671 (1963). (7) Miller, S. B., Lappin, G. R., and Tholstrup, C. E., Ultraviolet absorbers, Modern Plastics Encyclopedia, 45, (14A), 442 (1968). (8) Sandher, M. R., Hedaya, E., and Trecker, D. J., Mechanistic studies of the Photo-Fries reaction, J. Am. Chem. Soc., 90, 7249 (1968). (9) F6rster, T., Transfer mechanisms of electronic excitation, Discussions Faraday Soc., 27, 1 (1959). (10) Rauh, R. D., Evans, T. 1•., and Leermakers, P. A., Intramolecular energy transfer in some indole alkaloids and related donor-acceptor systems, J. Am. Chem. Soc., 90, 6897 (1968). (11) Leermakers, 1 . A., Byers, G. W., Lamola A. A., and Hammond, G. S., Intramolecular electronic energy transfer between nonconjugated chromophores in some model com- pounds, Ibid., 85, 2670 (1963) Ibid., 87, 2322 (1965).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)