J. Soc. Cosmetic Chemists, 20, 795-805 (Dec. 9, 1969) Bubble Formation in Hydroalcoholic Gels TONG JOE LIN, Ph.D.* Presented before the California Chapter, September 30, 1968, Los Angeles Synopsis--Formation of troublesome gas bubbles in cosmetic gels and viscous emulsions has been generally regarded as due to the entrainment of surrounding air during the processing of the products. It is proposed that, in the absence of external entrainment, gas bubbles can form in many cosmetic products through internal generation of gases. The internal genera- tion of a gas may be due to a chemical reaction involving evolution of a gaseous substance or a physical change involving a change in the solubility of the dissolved gas. The latter is believed to be responsible for gas bubble formation in many hydroalcoholic gels. A method of eliminating bubble formation due to such a mechanism is discussed. INTRODUCTION Presence of air bubbles in cosmetic preparations is generally un- desirable for various reasons. The trapped air can create pin holes and poor appearance in lipsticks or promote undesirable oxidation. The presence of air in creams or fluid make-ups can make the product texture appear coarse or can lower the specific gravity of the product to affect the net weight. It is known that the presence of excessive air bubbles in an emulsion can cause the emulsifier molecules to adsorb at the air-emul- sion interface and. deplete the emulsifier needed at the oil-water inter- face, resulting in poor emulsion stability (1). Control of aeration is a difficult problem in manufacturing many cosmetic products. Particu- larly if the product is viscous or has a high yield value, the trapped air may not escape and may remain in the product indefinitely (2). * Max Factor & Co., Hollywood, Calif. 90028. Present address: Chemical Works, 19 Fu Shin Road, Section 4, Taichung, Taiwan. 795 Shen Hsiang Tang
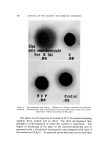
796 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS It is generally regarded that air bubbles in cosmetic preparations are incorporated into the products during manufacturing operations involv- ing entrainment of the surrounding air. However, very little work has been done in this area to determine the exact mechanism of aeration. Entrainment of the surrounding air into a liquid due to vortex forma- tion in a mixing operation is, of course, well-known and is probably the most common source of aeration in cosmetic preparations. In some cases, vortex formation may be desirable in order to facilitate wetting of a powdered material it is generally to be avoided since it promotes aer- ation and encourages evaporation of volatile components. When a pro- peller or turbine mixer is used, it is generally possible to eliminate the vortex by employing baffles or placing the mixer off-center (3). The surrounding air can also be entrained without a vortex if the fluid is in turbulent flow. The eddies and irregularities of the fluid sur- face produced by rapid mixing, pumping, or pouring operations can often be responsible for aeration. However, Lin and Donnelly have pointed out the possibility of air entrainment by smooth jets in laminar flow (4). Such possibility can exist in filling of viscous lotions using a circular noz- zleo Another possible source of air bubble entrainment occurs during a pumping operation when there is a leakage in the suction side of the line. However, this possibility, as well as others mentioned thus far, involves entrainment of the surrounding air and the air incorporated into the product originates externally. Although it is possible to produce gas bubbles in cosmetic products through internal generation of gases, there appear to be few publications concerning such a mechanism in practical situations. It is theoretically possible to generate a gas in a product either as a result of a chemical reaction liberating the gas or a decrease in dissolved gas solubility. Since most cosmetic materials are quite inert, it would be unusual for a chemical reaction to take place during the manufacturing operation or shelf-life resulting in liberation of a gas. However, internal generation of gas bubbles due to a decrease in solubility characteristics probably occurs more frequently in cosmetic processing than is sus- pected. Since the solubility of a gas usually decreases with temperature, heat- ing of a liquid saturated with a gas generally results in liberation of the dissolved gas. However, it is also possible to generate gas by mixing two liquids together under an isothermal condition. To explain, Fig. 1 il- lustrates a solubility curve of a gas, X, in a binary mixture of AB solu-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)