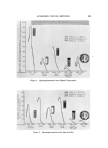
BUBBLES IN GELS 803 -• •'•-•. 7 ..... " ß • • .... • ...................... :• •" ":" '7., '•' :: • ..•'.•,. • ....... ........... . . - . ... ...,•.• ..... :. • -. . •:•-...• •......• . - ..• '• .•.z.:.. ß .: :'..'•" i.•: 2 .... • ..... .::.... ...:•:: ' .. Figure 4. Carbopol gels prepared by four different procedures (1 = control, 2 = delayed neutralization A, 3 = delayed neutralization B, 4 = ultrasonic method) only a very small amount of air bubbles and the gel from Procedure IV contained virtually no air bubbles. The photograph in Fig. 4 shows the samples taken from this series of experiments. The number on the bottle corresponds to the procedure used. From the results obtained, the theory of dissolved air appears to be a plausible explanation for the observed bubbles in Carbopol gels. The formation of such bubbles can be prevented in Carbopol gels by a delayed neutralization technique either with or without deaerating equipment such as an ultrasonic machine. However, the cavitation produced by an ultrasonic unit is very effective in removing the dissolved air and such a machine can be very useful in shortening the time required to free the undissolved air. In order to study the time required to free the undissolved air, another series of experiments was conducted with the same Carbopol system using Procedure III. The Carbopol resin was first dispersed in the alcohol using a propeller mixer. After all the bubbles in the dis- persion had escaped, water saturated with air was uniformly blended into the mixture in a large flask. The total weight of the mixture was 2 kg. Immediately, a 2-in. propeller mixer was placed in the solution to agitate the mixture at about 300 rpm. Samples of 100 g each were taken at various intervals after mixing was started and the neutralizer was added to these samples to produce gels. The mixture was covered during the mixing to prevent evaporation. These gel samples were placed in a constant temperature bath at 24 øC and their densities were determined after an equilibrium was reached.
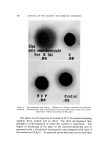
8O4 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS o .905 •BLE FREE / GEL. 50% ETHANOL 0.5ø/ø CARBOPOL 940 0.5% T.E.A. 49ø/ø WATER 60 120 MIXING TIME BEFORE NEUTRALIZATION (MIN ) Figure 5. Effect of delayed neutralization The results of these experiments presented in Fig. 5 indicate an increase in gel density (i.e., decrease in the amount of bubbles) with the mixing time before neutralization until a maximum value is reached. The graph shows that after 2 hours of mixing, practically all the air had escaped from the system as the gel sample gave a maximum density of 0.9176. Visually, this gel showed very few air bubbles. Since the releasing of the undissolved air is dependent on the degree of agitation, the temperature of the system, etc., the result presented in Fig. 5 cannot be directly used to predict the extent of deaeration in a large processing vessel. However, the data clearly demonstrate the effectiveness of the delayed neutralization technique. It is interesting to note that by coupling this technique with the use of an ultrasonic machine, the mixing time was reduced from 2 hours to 10 minutes in making a bubble- free gel. CONCLUSIONS Experimental data presented indicate that in the absence of ex- ternally entrained air, hydroalcoholic Carbopol gels can form bubbles depending on the method of gel preparation. There was strong evidence suggesting that the observed bubble formation resulted from internal liberation of the :undissolved air following a reduction in air solubility in the system. The Carbopol polymer itself did not appear to affect bubble formation except that, when neutralized, the gel trapped the liberated air to produce bubbles '
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)