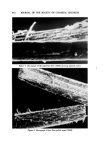
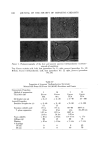
AEROSOL EMULSIONS AND FOAMS Table VII Stepwise Addition of Aqueous to Oil Phase and Vice Versa 635 Excess Triethanolamine System-Stepwise Addition of Triethanolamine to Mineral Oil/Myristic Acid Solution Proportion of Triethanolamine Added (wt %) 1/3 2/3 3/3 Emulsion Droplet Size Range (t•) • 2--5 • 2-10 • 2-30 Excess Myristic Acid System-Stepwise Addition of Myristic Acid/Mineral Oil Solution to Aqueous Triethanolamine Proportion of Emulsion Droplet Myristic Acid Added Size Range (wt %) 1/3 • 2-30 2/3 • 2-10 3/3 • 2--10 Figure $. Stepwise addition of aqueous TEA to mineral oil--myristic acid solution (excess TEA system) Top left. 1/3 TEA added right, 2/3 TEA added Bottom. 3/3 TEA added In the second experiment, involving the excess myristic acid system, the mineral oil/myristic acid solution was added in three equal portions to the aqueous triethanolamine solution. The data in Table VII and the photomicro- graphs in Fig. 6 show that the range of droplet size in the emulsion decreases
636 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Figure 6. Stepwise addition of mineral oil-myristic acid solution to aqueous TEA (excess myristic acid system) Top left. 1/3 MA added right, 2/3 MA added Bottom. 3/3 MA added as addition of the oil phase continues. This is because the concentration of the complex increases as with continued addition. The results also indicated that better emulsification was obtained when the aqueous phase was at room temperature during addition rather than 54.4øC. A possible explanation is that the triethanolamine myristate/myristic acid complex is decomposed at the higher temperature and does not form until the emulsion is cooled below the complex decomposition point temperature. The fact that the complexes decompose at higher temperatures has been reported by Epstein et al. (6). The decomposition point is referred to as the film drainage transition temperature. The worst emulsions were obtained when hot water was added to the hot mixture of triethanolamine, myristic acid, and mineral oil. Apparently, the complex does not form in mineral oil alone. The temperature probably is too high and, also, the ion-dipole and dipole-dipole interactions involved in com- plex formation do not take place in the oil phase. CONCLUSIONS 1. Emulsion concentrates with small droplet diameters and long creaming times produced better aerosol emulsions than inferior concentrates. The su- perior aerosol emulsions gave more stable foams with a smaller range of bub- ble diameters.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)