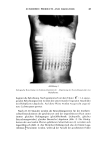
102 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 5O 4O % 30 •- 2o 5a RELATIVE UNITS OF LIMONENE '• 10.- 016 • ß 6/4 ••__ _•_____ •o ,am • -- 0 • ..... • ...... • ...... 0 1 4 16 64 LEVEL OF PULEGONE ADDED TO LIMONENE 5b RELATIVE UNITS OF PULEGONE ,,Xl ß q &•o 16 '0, 4- ..... 4, ..... ..... 4? d o 1 4 16 64 LEVEL oF LIMONENE ADDED TO PULE60NE Figure 5. 5a) Relation between the level of limonene and perceived citrus smell for various added levels of pulegone. 5b) Relation between the level of pulegone and perceived mintiness for various added levels of limonene. DISCUSSION The data discussed here from two studies illustrate several important points, both of substantive and methodological nature. Untrained and naive panelists can discern and successfully scale a large number of odorants. Their magnitude estimation ratings vs. known physical measures of concentration conform to lawful functions. The same panelists can assess other attributes (such as hedonics and quality) in the same session along with iudgment of odor intensity. Odor intensity conforms to a power function of concentration, with the exponent almost always less than 1.0. This indicates that large measured changes in odorant concentration produce smaller changes in perceived odor intensity. Furthermore, this also suggests that in the practical application of perfumery the consumer may be able to discern concentration differences quite well, but the consumer will also contract or shorten the magnitude of the change that the formulatot makes into a much smaller sensory change. The minimum perceptible difference cannot be estimated, however, from these scaling studies. Cain (1977) recently showed that increments of as little as 5% for pure odorants can be differentiated from each other. Evaluations from a moving airstream (dynamic method) produce perceptions of odor intensity different from parallel evaluations of the same odorant from a static source. This difference is critical for the odor intensity function. The function vs. concentration is much steeper (i.e., odor intensity grows more rapidly
PSYCHOPHYSICAL MEASUREMENT 103 with concentration) in a dynamic airstream than in an evaluation of odorants which are dissolved in only liquid and smelled from an unstoppered bottle. Odor intensity in mid-ranges of odor intensity can be moderately well predicted by the vector model of additivity, but only if the ratings exhibit ratio-scale properties. That is, in order to use the vector model (or a similar quantitative model to predict mixture strength) the experimenter should have the individual evaluate odor intensity using magnitude estimation, which produces the appro- priate sensory ratio scale. A scale with fixed limits (1-5, 1-9) is not adequate for predictive purposes. The comparable analogy is a fixed thermometer scale, between 0 and 100. Few rules of thermodynamics can be reduced using such a scale. Rather, a scale with ratio properties, such as the absolute of Kelvin scale, must be used. Odor hedonics can be described by one of three patterns: (a) no change in hedonic pattern with shifts in odorant concentration (b) monotonic (continuous) decrease in liking, with increasing concentration and (c) slight increase in hedonic tone with concentration, followed by a decrease (rarest form). Odor hedonics (liking/disliking) cannot be easily modelled by a linear combina- tion of the hedonic values of the unmixed components evaluated themselves. The pattern which emerges is that there is only a modest correlation between the hedonic tones of components, and of mixtures, even when a linear, weighted, regression model is used for prediction purpose. A nonlinear model, developed on an ad hoc basis, provides a better fit. Whether this means that the linear model is incorrect but that another more complicated model is appropriate, or whether there is really no appropriate model which will serve as a general predictor, remains for future studies. It is difficult, if not impossible, to predict the sensory levels of qualities for an odor mixture, a priori, from knowledge of the intensity of the qualities of their components. This is, the specific modifications in sensory character of odorants in mixtures are difficult to predict, a priori, when the two odorants are combined in noninteracting, vapor phase mixtures. Despite these limitations, the prospects appear bright for a further understanding of odor perception by psychophysics. Furthermore, because psychophysics deals with quantitative relations between physical and psychological aspects of odorants (as well as among stimuli for other senses), psychophysics may be a timely tool for applications in the cosmetic industry. No doubt, as cosmetic chemists become increasingly familiar with methods of psychophysical scaling and with the body of psychophysical literature on taste, smell, color and texture perception, psychophysics should see increasing service and applications in product development, product evaluation and product optimization. REFERENCES (1) S.S. Stevens, On the brightness of lights and the loudness of sounds, Science, 118, 576 (1953). (2) S.S. Stevens, In Pursuit of the Psychophysical Law, Second Public Klopsteg Lecture, Northwestern University, Evanston, Ill., 1%2. (3) B. Berglund, U. Berglund, G. Ekman and T. Engen, Individual psychophysical functions for twenty-eight odorants, Perception & Psychophysics, 9, 379-384 (1971).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)