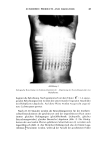
108 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Entropic Energy, E s Es is the energy contribution due to the steric interferences which bubbles experience as they approach one another. These contributions are not expected to be significant unless the film thins to a few nanometers thickness. This conclusion is particularly valid for surfactants in which the polar head groups are relatively small. For polymeric foam stabilizers and for nonionic surfactants, in which ethylene oxide or ethylene oxide/propylene oxide chains comprise the head group, the steric contribution is expected to be higher and to operate at longer distances. Electrical Energy, E• EE represents the energy contribution from coulombic forces. These interactions can be dominant in aqueous solutions of ionic surfactants. The electrical contribution for the case of spherical bubbles in aqueous solution at 25øC can be estimated using the equation of Hogg et al. (22,23). EE = 8.92 X 10 -•ø G •-2 [In (1 -3- exp (--KA)] where •' is the zeta potential of the bubble/solution interface, G is given by the formula, 1/G = 1/R• -3- l/R2, and K is the Debye-Huckel reciprocal length parame- ter in cm -• which is related to the total concentration, C, in mole/liter, of uni-univalent electrolyte in the solution by the expression, K = 0.328 X 10 -8 •/-•. The above equation applies for the condition of constant surface potential and nondeformability of the bubbles and assumes that the Gouy-Chapman model of the double layer interactions is applicable. The equation shows how the repulsive coulombic interaction increases as the bubbles come closer together and so opposes the film thinning. In summary, of the three energy factors involved, the coulombic and the steric energy factors will oppose thinning. The role of the van der Waals' term, Ev, is either to oppose or promote film thinning, depending upon the relative values of the Hamaker constants of the surfactant and the medium. The magnitude and the sign of all these energy factors are a direct function of film thickness, as is demonstrated in Figure 1, drawn on an arbitrary scale. Depending upon the relative values of each factor, two extreme possibilities can be foreseen. 1. The film thinning is favored energetically under all conditions, so that the film will finally rupture causing eventual foam collapse. 2. The film thins to a particular point beyond which further thinning requires crossing an activation energy barrier so that the film achieves a metastable equilibrium thickness. Further film thinning or film rupture requires an additional energy source which is capable of either altering the activation energy barrier or providing enough energy to overcome it. Corresponding to the above two conditions, two classes of foams are possible, viz., nonpersistent foams and persistent foams, respectively. In nonpersistent foams, film thinning is energetically always favored and the life span of the foam is primarily controlled by the rate of film thinning. On the other hand, in persistent foams, drainage and lamella thinning occur until an "equilibrium" film thickness, he, is reached. These foams are relatively stable and their breakdown is the result either of
ANTIFOAMS 109 z z ¸ z SCHEMATIC REPRESENTATION OF ENERGY PROFILE DURING FOAM THINNING EE / / ,, / • /- DISTANCE OF /' /ET TM • SEPARATION, Figure 1. Variation in the interactive energies on approach of two bubbles. Curve 1 shows a net positive energy barrier Curve 2 does not. external factors or of local thermal motions which can cause fluctuations in the film thickness to or below the critical rupture thickness, hr. The actual stability of the foam and the values of h e and h• are dependent on (a) the interfacial packing density of the stabilizing surfactant and (b) the nature, size and configuration of its polar group. The rate of film thinning depends directly upon the magnitude of the driving energy and inversely upon the bulk and surface viscosities. In particular, the role of surface viscosity becomes significant when appreciable film thinning has already occurred. Even nonpersistent foams can demonstrate remarkable stability by virtue of having very slow film drainage characteristics. This is particularly true for foams where the surface and/or bulk exhibit non-Newtonian behavior such that the viscosity increases sharply with a decrease in the shear rate or the drainage rate. Under these conditions, even though film thinning is favored energetically, the slow kinetics of thinning impart appreciable stability to the foam. These types of foam are expected to be encountered with nonionic surfactants or polymers in aqueous solutions or, in general, in nonaqueous media, where electrical repulsive forces play a limited role.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)