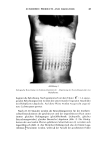
112 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 200 I I I I 70- ANTI FOAM' 160 ppm • CMC 0.001 0.01 0.1 1.0 SODIUM DODECYL SULFATE CONCENTRATION %(Wt/Wt) Figure 2. Antifoam effectiveness, r/, and zeta potential (•') of AF-1 as a function of SDS concentration. Surface tension plot of SDS without antifoam is included. -60.0 E --50.0 m z N - -40.0 surfactant, provided an important clue as to the mechanism of step two above (10). When the concentration of the ionic surfactant approached and increased through the CMC, a sharp drop in antifoaming efficiency was observed. At the same time, zeta potential measurements showed that the coulombic charge on the antifoam droplets underwent a rapid increase. The inferred sharp change in the repulsive interaction of the droplets around the surfactant's CMC is attributable to a marked increase in the adsorption of surfactant molecules on the antifoam droplets in this region. Such adsorption is expected to occur through interactions between the surfactant's hydrocarbon chain and the silicone oil droplet, leaving the ionic headgroup of the surfactant still exposed to the solution phase and thereby charging the droplets, as was observed. Under these conditions, the process of droplet movement to the bubble interface involves the transport of a charged droplet towards a similarly charged gas bubble, requiring considerable forces of coulombic repulsion to be overcome. This is clearly illustrated in Figure 2 where antifoam activity is plotted as a function of SDS surfactant concentration along with the SDS solution surface tension (before addition of antifoam), and the surface charge on the antifoam (measured by electrophoresis)--a measure of the repulsive interaction energy, Vm. The sharp change in the antifoam performance accompanying the sharp rise in repulsive interaction energy is quite evident. In addition to mechanical and diffusion forces, the transport of antifoam droplets from bulk to the interface is thus governed by coulombic and van der Waals' forces. In other words, there is a formal similarity to the bubble/bubble interaction scheme presented in the last section on foaming mechanisms, but it differs since the
ANTIFOAMS 113 interaction in this case is between dissimilar bodies and is therefore more complicated. Details of the calculation allowing the construction of energy/distance plots have been presented elsewhere (8) only the main conclusions will be given here. It was shown that, depending upon the test solution conditions, as an antifoam droplet approaches a foam bubble surface, it can experience either an attractive or repulsive interaction and thereby the antifoaming process will be directly influenced. In particular, in systems containing ionic foaming surfactants the repulsive interactions are important. As pointed out before, the repulsive interaction depends not only on the nature of the surfactants but also on their concentration. It is related (8) to the potential and distance of the particle (subscript 1) and bubble (subscript 2) by the expression EE = 2.23 7K 10 -•ø R•(•-•24-•'•2)( 2P (14-exp(-- KA)) ) ' (1 4- p2)In (1 -- exp(-- KA)) 4- ln(1 -- exp(-- 2KA)) where the symbols have the same significance as in the previous expression for EE and P is the zeta potential ratio (•'•/•'2). The role of van der Waals' interactions is more complex and is described in detail in the aforementioned publication (8). In short, these interactions will favor antifoaming if the Hamaker constants of the surfactant (As), antifoam droplet (ap) medium (A,,) are such that A,, • As and Ap, or A m • A5 and Ap. However if A5 A m • Ap, or Ap • A m • As, then one expects an overall repulsive van der Waals' interaction which would diminish the foam inhibition. Having considered some of the factors governing transport of the antifoam droplet to the bubble interface, it is now appropriate to consider the forces involved during entry into the interface, spreading and rupture. The first two of these are governed ultimately by short range, interfacial energies, as can be inferred from the definition of the so-called "entering" coefficient (e) of the droplet into the interface and its "spreading" coefficient (s) along the interface (19): e=7w--70 4-7ow S=7w--70--7ow where 3/w is the surface tension of the foaming solution, 3/0 is that of the antifoam liquid equilibrated with this solution, and 3/ow is the interfacial tension between the two equilibrated liquids. For effective antifoaming to occur it is a prerequisite that the antifoam liquid spread spontaneously at the bubble interface, i.e., that s be positive. If s is positive, e must also be positive, i.e., entry of the droplet into the interface is automatically facilitated. A positive spreading coefficient is favored by a high 3/w value and low values of 3/0 and 3/ow. The first and third terms, viz., 3/w and 3/ow, are both reduced by the presence of (foaming) surfactant it is the relative effect of the surfactant which is important. It is, however, a matter of experience that the spreading of a liquid tends to decrease as the surface tension of the aqueous subphase is decreased. This fact is in line with our previous statements concerning the requirements of antifoams, which, in fact, become more rigorous the lower the surface tension of the foaming solution. One of the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)