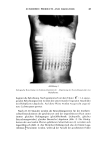
114 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS principle attributes of silicone liquids is their ability to spread on virtually all aqueous surfactant solutions this ability is directly connected with their low surface tension ('¾o• 20 dynes/cm). We come now to the last step involved in the antifoaming process, i.e., bubble rupture. In silicone oil-based antifoams it has been clearly shown that the presence of hydrophobic filler particles markedly improves the defoaming ability of the antifoam fluid. It is now believed, with some experimental supporting evidence, that these finely divided hydrophobic filler particles directly participate in the process of bubble rupture. Thus, in the five listed steps of antifoaming, the first four are primarily affected by the properties of the antifoam fluid, but the final step involves the filler in the following way: once the antifoam droplet enters the bubble and spreads, the AIR III!1111111111 SOLUTION /•.•-- HYDROPHOBIC SILICA PARTICLE 3 SOLUTION '• IIIII SOLUTION AIR SOLUTION IIII SOLVATED SILICA PARTICLE RUPTURE Figure 3. Schematic representation of the mechanism of bubble film rupture by hydrophobic silica particles.
ANTIFOAMS 115 hydrophobic filler particles are swept along the interface. The phenomenon of bubble rupture is explained by the adsorption/depletion of foam stabilizing surfactant molecules by the filler, so causing these particles to become hydrophilic, and ultimately to be extracted into the aqueous phase, as represented in Figure 3. Thus a net transfer of silica from antifoam phase into the aqueous phase is expected and this has, in fact, been experimentally observed (9). It is postulated that if the local depletion of surfactant at the interface is rapid, the resulting surface chemical shock will render the bubble unstable and rupture will occur. The overall picture which emerges from the above analysis is that, while the hydrophobic silica filler acts as the actual foam breaker, the silicone oil acts as a carrier fluid which prevents the filler particles from coming into contact with surfactant molecules, in this way maintaining their hydrophobicity until they are brought to the point where they exercise their potent antifoaming activity, i.e., in the surface of the foam bubble. The uniqueness of silicone oil lies in its inertness, in its ability to spread spontaneously on most aqueous fluids and in its ability to maintain the form and size of its droplets in solutions when suitability stabilized. The ability of silica filler particles to act as the foam breaker is primarily dependent on their hydrophobicity and high surface area. A higher degree of hydrophobicity will not only promote potency of the filler particle but will also aid dispersion in the silicone oil. Thus the combination of silicone oil and highly hydrophobic silica particles forms an all-purpose antifoam which can perform effectively in most foaming systems. It is important to mention that the above mechanism has been developed for filled antifoam systems. A different mechanism of action is expected for untilled antifoams. CLASSIFICATION AND CHARACTERIZATION OF FOAMS AND ANTIFOAMS From the practical viewpoint of antifoaming, foams can be classified in two main ways based on the nature of the stabilizing entity and on the actual mode of foam stabilization. With respect to the former there are five main groups of stabilizers: (a) ionic surfactants, (b) nonionic surfactants, (c) mixed surfactants, (d) polymers, including proteins, and (e) particulate stabilizers. As will be shown later, knowledge of the type of surfactants present facilitates the choice of the antifoam, but it provides only partial mechanistic insight into a potential antifoam's performance. As regards the second classification, four stabilization modes can be listed, namely: (a) by electrical charge, i.e., repulsion force, (b) by entropy, i.e., steric forces, (c) by viscosity, i.e., control of drainage rate, and (d) by a combination of the preceding stabilization mechanisms. In addition to the above-mentioned classification of foams, there are several specific foaming media characteristics which should be known in order to predict the performance of added antifoams and facilitate their choice. These characteristics are: (1) the state of the foaming system, i.e., its homogeneity, (2) the solution viscosity, (3) the surfactant concentration in relationship to the critical micelie concentration (CMC), (4) the surfactant type, i.e., ionic, nonionic or mixed, (5) % the solution surface tension, i.e., low ('38 dynes/cm), medium (38-45 dynes/cm) or high (:45 dynes/cm), and (6) the operating temperature. These factors will be elaborated upon
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)