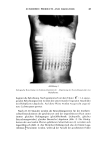
116 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS later. However we stress here that the state of the foaming system is important because heterogeneity of the foaming solutions, as brought about by suspended solids, can accelerate antifoam loss from the system through adsorption or coalescence. This will eventually result in lower antifoam performance. At the same time, suspended solids can sometimes aid foam stabilization and thereby interfere with the antifoaming process. We note here also that the relevance of solution viscosity in antifoaming comes from the fact that, in addition to affecting the rate of foam drainage, it will influence the transport of the antifoam droplets to the interface--a vital step in the overall antifoaming process. A general classification of antifoams is presented in Figure 4. As seen in this compilation, there are two main types of antifoams, viz., (1) filled and (2) untilled antifoams. Filled antifoams refer to those which comprise an antifoam base fluid and a small amount (generally 3-10% by weight) of finely divided inorganic filler. In most cases the filler is a fumed silica such as Cab-O-Sil (Cabot) having an average particle GENERAL CLASSIFICATIONS OF ANTIFOAMS (Represents Broad Guidelines Only) ANTIFOAM TYPE SILICONE ORGANIC MIXED ANTIFOAM COMPOSITION* WHERE EFFECTIVE** FILLED ANTIFOAMS so, sio 2 MO, SiO 2 M0/SO/Si0 2 A, B, C B, C, B, C, ANTIFOAM COMPOSITION* WHERE EFFECTIVE** UNFILLED ANTIFOAMS S-G PG SO, S-G, PG C C B, C SO = Silicone Oil SiO 2 = Silica, MO = Mineral Oil S-G = Silicone-Polyglycol Copolymers PG = Polyglycol (e.g., PPG) A: Ionic Surfactant, High Conc., Low S.T. B: Ionic Surfactant, Low Conc., Nonionic Surfactant Solution, Medium-Low S.T. C: Solutions of Polysaccharides, etc., High-Medium S.T. Figure 4. Generalclassification ofanti•ams.
ANTIFOAMS 117 size from 100-2000 • and a surface area of 50 to 350 m2/gm. The base fluid is generally a nonpolar fluid such as mineral oil or a polydimethylsiloxane oil without any pendant groups. Incorporation of silica markedly improves the antifoaming properties of the base fluid. These antifoams require predispersion for effective action. Unfilled antifoams, on the contrary, are self-dispersible by virtue of the presence of a hydrophilic-hydrophobic group combination in the molecule. Such antifoams can be highly effective, but only in specific systems they also tend to be inexpensive. In each of the above two categories there are antifoams made either from silicone fluids, organic fluids or mixed fluid systems. The choice of the particular fluid system, of course, depends upon the nature of the foaming system and the type of control intended. In the antifoam classification of Figure 4, each of the listed categories is accompanied by a typical foaming system in which the antifoam will perform efficiently. The listing reflects solely performance and not necessarily cost effective- ness. Note, for example, that the silicone based antifoams are effective in a high concentration anionic foaming system, such as 0.5% sodium dodecyl sulfate, whereas antifoams belonging to the other categories will either be ineffective or less effective in defoaming this system. However for a protein stabilized foam all of the antifoams, including silicone/silica, are listed as being effective but will naturally have different efficiencies. SILICONE ANTIFOAM PREPARATION There are two major steps in the preparation of silicone antifoams: (a) preparation of the active mixture and (b) processing of the active mixture to a stable form. The active mixture preparation requires dispersion of hydrophobic fumed silica or other finely divided solid in silicone oil. This can be done by mixing prehydrophobized solid filler with the fluid or, alternatively, by mixing hydrophilic filler and hydropho- bizing it either by heat treatment in situ or by incorporation of a hydrophobizing agent followed by heat treatment. As stated earlier, the active mixture so produced is unsuitable for direct use owing to its poor dispersibility. Therefore the second step in the antifoam making process is the conversion of this compound into an aqueous emulsion or otherwise appropriately formulated form. Proper emulsification of these silicone antifoams is necessary for their overall effectiveness. Nonetheless, the preparation of an acceptable grade of emulsion is still an art and is successfully practiced by only a few companies. Note: AF-1 and TAF-1 are designations for representative conventional and transient antifoam types, respectively, and will be used throughout this report to illustrate performance variations in different foaming systems. ANTIFOAMING TESTING Depending upon the particular end application, several antifoam tests are in use today. Most of these tests are, however, qualitative and, although generating sufficient information for the intended purpose, do not provide fundamental insight into the phenomenon of antifoaming. For the latter purpose, the "nitrogen bubbling" test is
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)