FORMULA OPTIMIZATION 75 optimization method within the optimum area or to perform a sophisticated statistical analysis on all the points actually measured. REFERENCES (1) G. E. P. Box and K. B. Wilson, On the experimental attainment of optimum conditions, J. Royal Statist. Soc., series B, 13, 1-45, (1951). (2) J. A. Nelder and R. Mead, A simplex method for function minimisation, Comput. J., 7, 308-313 (1965). (3) V. Tran, J. L. Morat, and D. Bertrand, A new serial method for searching the optimum operating conditions of a technological process, J. Chemometr., 5, 73-84 (1991). (4) M. J. Box, A new method of constrained optimization and a comparison with other methods, Comput. J., 8, 42-52 (1965). (5) M. J. Box, A comparison of several current optimization methods and the use of transformations in constrained problem, Comput. J., 9, 67-77 (1965). (6) G. E. P. Box, W. G. Hunter, and J. S. Hunter, Statistics For Experimenters (John Wiley & Sons, New York, 1978). (7) G. E. P. Box and N. R. Draper, Empirical Model Building and Response Surfaces (john Wiley & Sons, New York, 1987).
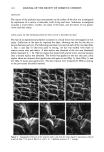
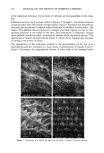
J. Soc. Cosmet. Chem., 45, 77-84 (March/April 1994) Secondary structural rheology of a model cream LORRAINE E. PENA, BARBARA L. LEE, and JAMES F. STEARNS, Drug Delivery R&D--Specialty Products, The Upjohn Company, Kalamazoo, MI 49007. Received November 18, 1993. Presented at the Midwest Regional Meeting of the American Academy of Pharmaceutical Scientists, Chicago, May 1989. Synopsis The rheology of a cream is determined by the structure formed by its ingredients. The primary structural rheology of O/W creams has been characterized by the gel network theory. Cetyl palmitate is frequently used to improve consistency, but its mechanism of action has not previously been determined. This study uses rheometry and thermal optical analysis to examine the structural rheology of a model cream containing cetyl palmitate. Rheograms were obtained over the temperature range 25ø-40øC. In general, the overall rheogram shape, indicative of the primary structure, changes little as a function of temperature. However, an inflection gradually disappears as the temperature increases. Photomicrographs of the melting sequence show the cetyl palmitate embedded in the emulsifier network and illustrate the destruction of the cetyl palmirate secondary network structure. Photomonitor recordings correlate the melting transition of the cetyl palmirate with the disappearance of the rheogram inflection. INTRODUCTION The flow properties of semisolid dosage forms are determined by the structure formed by their ingredients. Creams generally consist of emulsifiers, fatty amphiphiles, waxes, oils, and water. The basic structure of O/W emulsions has been well characterized by the gel network theory (1-3). According to this theory, the oil phase is mechanically entrapped by a liquid crystalline gel network structure formed in the continuous phase by the surfactant and a fatty amphiphile. The surfactant and fatty amphiphile combine in definite ratios specific to the system, and the consistency of the cream is adjusted by varying the concentration of this fixed ratio combination. Commercially available emul- sifying waxes such as the Lexemul © and Promulgen © series are basically precombined surfactant/fatty amphiphile systems. In the absence of an oil, these systems are designated ternary systems and exhibit physical properties similar to those of their corresponding emulsions. In fact, the basic rheology of these ternary systems controls the rheology of the final cream (3). Although the basic structure of creams has been well characterized by the gel network theory, the role of other ingredients, such as cetyl palmitate, that add "body" to commercial creams has not previously been determined. A model cream is used in this study to demonstrate the role of cetyl palmitate in structure development. 77
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)