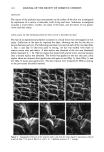
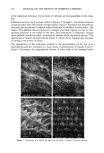
j. Soc. Cosmet. Chem., 45, 109-118 (March/April 1994) A two-stage method for the in vivo replication of human skin: Method refinement and application KIMBERLY L^FRENIERE and ROBERT DIFRUSCIA, DowBrands L.P., I603 Building, Midland, MI 48674 (K.L.F.), and Dow Chemical Canada, Inc., Vidal St. S., P.O. Box 3030, 63 Building, Sarnia, Ontario, N7T 7MI, Canada (R.D.F.). Received April 29, I993. Synopsis A two-step replication process that utilizes Reprosil and Spurr's embedding medium has been critically evaluated for use in the study of personal care products on human skin. The high dimensional stability of the Reprosil material makes collection of entire data sets possible. This is especially convenient in multiple- person studies, where all samples may be collected and processed together under similar conditions at a laterdate or be sent collectively to an independent testing laboratory. The replication process is particularly well suited for the replication of fine, delicate structures. Details of hair follicles and the surface of leaves were excellently reproduced. The replication process may be easily and conveniently applied to the study of effects of personal care products on human stratum corneum, as long as the identical testing site is utilized for before-and-after comparisons. INTRODUCTION The study of the effects of personal care products on human stratum corneum has been facilitated by replication of the in vivo state with subsequent topographical analysis by scanning electron microscopy (SEM). Indeed, in 1976, Garber (1) employed replication techniques with SEM to evaluate the effects of moisturizing soaps, abradent cleansers, moisturizing hand lotions, and other active ingredients on human stratum corneum. Garber employed an RTV-11 silicon resin to make the negative replica and a thermo- plastic resin that was either fine spray-dried or as-polymerized polyolefin "bead" poly- mer to make the positive impression. In 1979, Pameijer (2) evaluated the dimensional integrity of a variety of dental impression materials in combination with different negative impression materials. Pameijer concluded that the combination of Reprosil dental impression material and Spurr's low-viscosity embedding medium resulted in the best reproduction of detail, greatest versatility, and superior dimensional stability as compared to other materials tested. In this study, the suitability of the combination of Reprosil dental impression material and Spurr's low-viscosity embedding medium to the replication of human stratum corneum for the purpose of documenting the effects of personal care products was critically evaluated. Issues that include ease of application of 109
110 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS the method, dimensional stability of the negative replica, and ability of the method to reproduce fine, delicate structures were addressed. The method is applied to the deter- mination of the fate of an experimental polymer contained in a facial lotion applied to the forehead. EXPERIMENTAL REPLICATION The in vivo skin replication method involved a two-step process, as illustrated in Figure 1. The first step consisted of making a negative impression of the surface of the skin with a rubbery vinyl polysiloxane material, Reprosil (Dentsply International, York, PA). The second step consisted of producing a positive impression from the Reprosil negative using a low-viscosity epoxy resin, Spurr's embedding medium (Electron Microscopy Sciences, Fort Washington, PA). To make a negative impression of the skin, the following procedure was used. For experimental procedures utilizing replication methods to study skin surface topography, it was imperative that the identical site on the skin be replicated each time. Ink from a ball-point pen was easily transferred to the Reprosil impression material from the skin. A mark (e.g., an arrow) was made on the surface of the skin under study with a ball-point pen. The mark was refreshed before each replica was made. For long-term studies, participants were asked to refresh the mark each day. The Reprosil was prepared as described in the instructions contained in the kit. The mixed material was applied to the area of skin under study, as a thin film, approximately 1 to 2-mm thick. A one-inch-diameter circular piece of 3.2-mm-thickness Teflon polymer sheet was next applied to smooth out the replica and provide a flat surface necessary for subsequent REPLICATION PROCESS SKIN REPROSIL NEGATIVE EPOXY • POSITIVE Figure 1. Schematic of skin replication process.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)