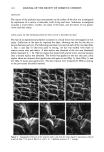
82 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS initial deviation from baseline at 36øC, which arrests at 50øC, begins a gradual ascent again at 56øC, then forms a final arrest at 60øC. Each of these arrests can be correlated with the visual observations. Upon examination of the photomonitor recordings of the raw materials, it is apparent that the arrests in the cream recording also correlate with the melting point of the individual raw materials. Cetyl palmirate (Figure 3B) begins melting at 3 IøC and completes the transition at 5 IøC, while Lexemu! AR © (Figure 3C) melts over the range 50-60.5øC. The comelt of these ingredients (Figure 3A) gives a recording showing arrests near the melting point of the individual ingredients, thus indicating that the components remain as distinct entities rather than coprecipitating as a solid solution. The first transition of the comelt begins at 28øC, is completed at 48.5øC, and is then followed by the second transition, which begins at 53øC and is completed at 58øC. These transitions correspond to the melting of the cetyl palmirate and Lexemu! AR © components, respectively. Based on the melting behavior of the raw materials and the comelt, it can be concluded that the wax particles in the first melting transition of the cream are cety! palmirate and that the higher melting structures are Lexemu! AR ©. Thus, the network structure of the cream consists of individual cety! palmirate particles dispersed throughout the matrix formed by the Lexemul AR © ternary system. As is apparent from the rheograms and x i values, the cetyl pa!mitate forms a secondary network structure within the cream, which is very sensitive to thermal changes and gradually disappears as the temperature is increased over the range 25-40øC. The thermal destruction of this network structure can be explained upon closer examination of the melting behavior of cetyl pa!mitate. As the melting transition in Figure 4 indicates, the cety! palmirate particles gradually decrease in their overall dimension as the temperature increases. Of particular note is that in response to the dimension reduction, the number of crosslinks between particles is substantially reduced at 40øC in comparison to 25 øC and that the crosslink destruction accelerates as the melting point is approached. The photomonitor recording indicates that the melting transition of cetyl palmirate begins at 3 IøC. Thermal optical analysis has shown that as part of the network structure of the cream, the cety! pa!mitate maintains its individual chemical integrity and follows the same melting transition. However, the particles are smaller than those pictured in Figure 4, and network crosslinks occur by contact with the Lexemu! AR © matrix particles as well as by self-association. The photomonitor recording of the cream indicates initiation of the cety! palmitate melting transition at 36øC rather than at 31øC, which would be ex- pected. This is probably an artifact of the limits of the microscope magnification, the lower birefringence of the smaller wax particles, and the limits of the photomonitor sensitivity. In other words, the actual initiation of the cety! pa!mitate melting transition in the cream is probably 3 iøC, but the limits of resolution of the instrumentation do not allow detection until 36øC because of the smaller particle sizes being observed. Thus, the thermal destruction of the cetyl palmirate secondary structure in the cream follows the same sequence as cetyl palmirate alone and is due to crosslink destruction in response to particle dimension reduction. CONCLUSIONS According to the gel network theory, the oil phase is mechanically entrapped by the
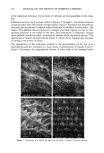
MODEL CREAM RHEOLOGY 83 Figure 4. Photomicrographs of the cetyl palmitate melting transition at 250 x magnification. A. 25øC. B. 40øC. C. 45øC. D. 49. IøC. liquid crystalline gel network formed by the emulsifier system. The cetyl palmitate behavior in the model cream is consistent with this theory. Hot stage photomicroscopy and thermal optical analysis have shown that cetyl palmitate assumes the role of the internal phase in an O/W emulsion. The photomicrographs indicate that the cetyl palmitate forms solid wax droplets that are surrounded by the emulsifier wax matrix. Photomonitor recordings show that cetyl palmitate and the emulsifier remain separate and distinct chemical entities in the cream. The gel network theory also states that the basic rheology of a cream is controlled by the emulsifier network. The rheology studies on the model cream show this to be the case also, but with an additional contribution from the cetyl palmitate. The structure determination of this investigation indicates that the inflection in the up curve of the rheograms is due to a secondary network structure formed by the cetyl palmitate and that the changes observed can be correlated with its melting behavior. As the melting process of the cetyl palmitate shows, increasing temperatures result in smaller particle dimensions and a reduction in the number of crosslinks. The gradual disappearance and shift of the rheogram inflection to lower shear rates are consistent with a network having a reduced number of crosslinks and a smaller particle size. At 40øC, there are evidently a sufficient number of crosslinks broken by the melting process that the secondary network structure no longer exhibits any greater resistance to shear than the primary structure formed by the Lexemul AR ©.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)