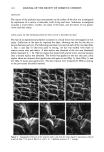
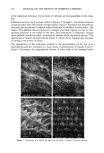
92 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS had been dyed with Orange II at pH 1 (hydrochloric acid) and at pH 7 (phosphate buffer) and then dried overnight (see Figure 2 and Table V). Figure 2 illustrates the droplets of water on three pieces of treated wool fabric after 60-second dyeing, rinsing, and drying overnight. The wool fabric dyed at pH 1 is clearly more hydrophobic than the wool fabric dyed at neutral pH. It is also more hydrophobic than the control fabric washed with 10% sodium lauryl sulfate and rinsed with water and then dried. The data of Table V summarize numerically the effects illustrated in Figure 2, and it demonstrates that wool fabric treated with an anionic dye (Orange II) at acid pH does make the fiber surface less wettable by water than does the same wool dyed at neutral pH. Furthermore, dyeing at a higher dye concentration (5% vs 0.5%) at neutral pH not only causes more dye binding (darker orange), but it also makes the fabric even more wettable. These findings suggest that the adsorption of anionic dyes and presumable anionic surfactants to keratins at acid pH (pH 1) is largely a charge-driven process. Thus the dye binds to the fibers primarily through electrostatic forces, and therefore, the hydrophobic portion of the dye projects outward, creating a hydrophobic hair fiber surface. However, as one increases the pH to neutral, the mechanism of action changes, and it becomes a hydrophobically driven process. As a result, the hydrophobic portion of the dye binds to the fibers, and the hydrophilic part of the dye projects outward, thus creating a more hydrophilic fiber. ORANGE-II {- ORANGE-II pH '•_1_ [0.5%] pH 77' [5.0O/o] pH ?_ •_0.5 •/o'• Figure 2. Illustration of the difference in wettability of keratin fibers after treatment of wool swatches with Orange II dye at acid and neutral pH.
ADSORPTION TO KERATIN SURFACES 93 Table V Orange II and Wool Fabric Wettability Dye concentration pH Wetting time (sec) 0.5% 1 2500.00 a 0.5% 7 29.6 -- 7 35.0 b 5.0% 7 12.6 a Significantly different by Kruskal Wallis test. SLS-washed control fabric. These results are consistent with the charge to hydrophobic continuum hypothesis for the adsorption to keratin surfaces. For the adsorption of cationic surfactants to hair fibers as a function of pH, one would expect the reverse effect, i.e., one would expect primarily ionic bonding at neutral pH (above the isoelectric of hair), which would render the hair surface more hydrophobic than at acid pH, where bonding would be more hydrophobic, thus creating a more hydrophilic fiber surface. The data of Table VI summarizes wetting times for wool fabric after treatment with CTAC at acid and neutral pH. These data are consistent with the predictions offered by the charge to hydrophobic continuum hypothesis, i.e., treatment at neutral pH creates a more hydrophobic fiber surface than treatment at acidic pH. One important and still unanswered question is: What is the nature of the adsorbing species? Is the adsorbing species a simple molecular species or is it an aggregate or a complex? In the case of simple solutions of cationics, e.g., CTAC, at low dilution, it is likely that molecular adsorption occurs. In more complicated systems, particularly in emulsions and suspensions, the adsorbing species may be a self-aggregate or some sort of complex formed between two or more different components. An example of the latter adsorption may occur in some conditioner systems containing long-chain alcohols and long-chain quaternary ammonium compounds. In this case, liquid crystals consisting of alcohol and quat can form at low concentrations as a result of reduced repulsion between ionic head groups in the quaternary compound when alcohol is interposed (11, 12). It seems likely for these types of systems that at least some alcohol-quat complex is adsorbed to the hair. In the case of cationic polymers or surfactants in an anionic surfactant medium, i.e., some conditioning shampoos, it is highly likely that complex aggregates of cationic and anionic species, and in some formulations, complex aggregates of cationic, anionic, and lipid components, adsorb to the hair. Table VI CTAC and Wool Fabric Wettability pH Wetting time (sec) 1 107.4 • 7 720 • Significantly different by Mann Whitney U test (p = 0.01 level).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)