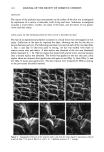
84 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The inflection can be treated as a secondary spur formation with yield value q'i. By definition, spur formations and yield values are associated with three-dimensional net- work structures (2). The thermal optical analysis studies have verified the three- dimensional nature of both the primary and secondary network structures of the cream formed by the Lexemul AR © and cetyl palmitate, respectively. REFERENCES (1) G. M. Eccleston, The microstructure of semisolid creams, Pharmacy International, 63-70 (March 1986). (2) B. W. Barry, "Rheology of Pharmaceutical and Cosmetic Semisolids," in Advances in Pharmaceutical Sciences, H. S. Bean, A. H. Beckerr, and J. E. Carless, Eds. (Academic Press, New York, 1974), Vol. 4, pp. 1-72. (3) G. M. Eccleston, The influence of fatty alcohols on the structure and stability of creams prepared with polyethylene glycol 1000 monostearate/fatty alcohols, Int. J. Cosmet. Sci., 4, 133-142 (1982).
J. Soc. Cosmet. Chem., 45, 85-94 (March/April 1994) Adsorption to keratin surfaces: A continuum between a charge-driven and a hydrophobically driven process C. R. ROBBINS, C. REICH, and A. PATEL, Colgate Palmolive Company, 909 River Road, Piscataway, NJ 08854. Received September 3, 1993. Presented in part at the 8th International Hair Science Symposium, Kiel, Germany, September 1992. Synopsis A hypothesis is presented that explains and clarifies a wide variety of experimental observations regarding the adsorption to keratin fiber surfaces. It explains that the mechanism of the adsorption of conditioning agents, dyes, and other substances to hair fibers can be considered as a continuum between a charge-driven process and a hydrophobically driven process and that the exact nature of the reaction depends primarily on the structure of the adsorbing species and the pH of the system. Several examples are provided that illustrate and provide support to this hypothesis. INTRODUCTION The adsorption of conditioning agents to human hair has been studied for decades (1-3), and although it is generally recognized that several variables contribute to deposition, the mechanism of action is generally explained on the basis of a charged interaction providing the primary driving force (1,5). However, with the relatively recent advent of 2-in-1 conditioning shampoos that employ highly water-insoluble dispersed silicones as conditioning agents, hydrophobic factors, which lead to the separation of a water- insoluble conditioning phase during rinsing, and Van der Waals forces, which cause large particles to adhere to surfaces, are clearly becoming more important to mechanistic discussions (4,6). A hydrophobic role in the adsorption process has been appreciated for decades since the early demonstration of increased adsorption to keratins with increasing carbon chain- length for both anionic surfactants (6) and for cationic surfactants (1,3). Thus it occurred to us that some systems might adsorb to hair surfaces via a process that is largely charge-driven and others by a hydrophobically driven process, but that as structural modifications are made to the adsorbing species or as the pH of the adsorption medium is changed, the resultant altered nature of the adsorbing species or of the keratin itself might affect the mechanism to make it less charge-driven or more hydrophobically driven or vice versa. As a result, we hypothesized that adsorption to hair might be considered a continuum (see Figure 1) between a charge-driven process and a hydro- 85
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)