j. Soc. Cosmet. Chem., 45, 183-192 (July/August 1994) Damage of hair fibers as evaluated by an electrical capacitance technique HIRAKU ITO, YOICHIRO MURAOKA, and HARTWIG HOCKER, Heian Jogakuin (Saint Agnes') College, Nampeid•i, Takatsuki, Osaka 569, Japan (H.I., Y.M.), and Deutsches Wollforschungsinstitut, Veltmanplatz 8, Aachen D-S100, Germany (H.H. ). Received September 24, 1993. Synopsis An attempt was made to evaluate the damage of hair fibers using an electrical capacitance technique, and the results were compared with those obtained by using the Wilhelmy principle. To this end, two types of hair fiber samples were prepared. In one type, hair fibers were oxidized with dichloroisocyanuric acid (DCCA), and in the other, the fibers were physically rubbed with sandpaper. Water transport along the hair fiber bundle was examined with an apparatus using an electrical capacitance technique. The rate of water transport increased with the degree of damage. These results were consistent with the results on the contact angle measurements carried out with a Wilhelmy-type apparatus for recording the wetting force of a single hair fiber in water. Therefore, we found that an electrical capacitance technique is potentially useful as a convenient evaluation of the damage to hair fibers. INTRODUCTION Human hair fibers are treated repeatedly in many processes such as bleaching, dyeing, and permanent waving. As a result, hair fibers accumulate damaging effects. A method to diagnose the degree of damage of hair fibers would be very beneficial. However, no "convenient" method has yet been developed for evaluating the damage to hair fibers. As is well known, keratin fibers such as hair and wool have a so-called skin-core structure. The core, i.e., the cortex, is hydrophilic in nature due to the large number of polar groups contained in the polypeptide chains. On the other hand, the surface of keratin fibers is water-repellent, since the surface layer of cuticular cells (epicuticle) is hydrophobic in nature (1). Furthermore, there is a non-polar layer of fatty acid on the surface (2). When the keratin fiber is damaged by physical and/or chemical treatment, the structure of the fiber surface is modified. As a result, the surface property of the fiber is changed from a hydrophobic to a hydrophilic one. Therefore, the degree of fiber damage might be expected to correlate with the hydrophilicity of the fiber surface. Recently, we developed an apparatus using an electrical capacitance technique for eval- uating the water transport behavior along fiber bundles consisting of a few textile fibers 183
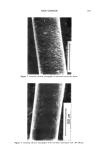
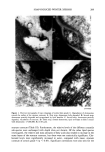
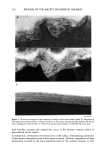
184 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (3). We found that there was a minimum (critical) number of fibers needed for water transport to occur, depending on the wettability of the fiber samples. That is, the water transport behavior of fiber bundles seems to be highly dependent on the apparent hydrophilicity of the fiber surfaces. In this study, we attempted to develop a convenient method for evaluating the degree of damage to hair fibers by observing the water transport behavior along fiber bundles by using an electrical capacitance technique. We compared the results with those of the dynamic wetting forcer measurements on a single hair fiber in water using the Wilhelmy principle (4,5) in order to ascertain the reliability of our method. EXPERIMENTAL SAMPLE MATERIALS Human hair fibers were obtained from a Japanese girl, age 15. All samples were cl•eaned with nonionic detergent solution, and then Soxhlet-extracted successively with a mix- ture of chloroform/methanol (50/50 vol) and diethylether. Surface modification of the human hair fibers was effected by two methods: chlorination with dichloroisocyanuric acid (DCCA) and physical rubbing with sandpaper. Ch[orina- tion was carried out according to the method described by Makinson, who applied it to wool fibers (6). Hair fibers (0.5 g) were immersed in aqueous DCCA solution (50 ml) containing 0.05% of nonionic detergent at pH 6.5 and at 7øC for two minutes. The fibers were then treated with dilute HC1 solution (pH 2.5) for one minute, followed by treatment with 2% sodium hydrosulfite (pH 4.5) for one minute and with 1% sodium bicarbonate at pH 7.5 for one minute, and finally rinsed with deionized water (three times) and dried. Three levels of treatment were applied (3, 6 and 10% DCCA). Physical rubbing was carried out by rubbing a hair fiber against No. CC-1500. sand- paper. Amino acid analyses and scanning electron microscope observations were carried out as described in a previous paper (7). WATER TRANSPORT MEASUREMENT (3) Water transport along a hair fiber bundle was measured using an electrical capacitance technique. A detailed explanation of the apparatus and of the procedure have been given previously (3). The length of the horizontally placed sample hair fiber bundle was 4 cm. CONTACT ANGLE MEASUREMENT The liquid-solid contact angle between water and a single hair fiber was evaluated by measuring the wetting force (F w) according to the Wilhelmy principle (4,5). Wetting force. When a solid is partially immersed in a liquid, the liquid either rises or is depressed along the vertical wall of the solid, thus exerting a force on the solid. The vertical component of this attraction force is the wetting force (Fw), Fw = •tvPcos 0 (1) where y LV is the surface tension of liquid (dyne/cm), P is the perimeter of the solid
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)