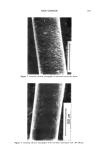
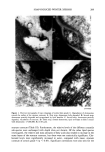
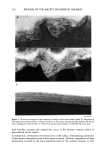
192 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS CONCLUSION The degree of damage of hair fibers was determined using an electrical capacitance technique, and the results were compared with those obtained using the Wilhelmy principle. The water transport behavior along a hair fiber bundle is strongly influenced by the damage. In addition, the degree of influence depends on the degree of damage to the hair fibers. Therefore, the method of water transport measurement along a hair fiber bundle using an electrical capacitance technique is useful and convenient in eval- uating the degree of damage to hair fibers. ACKNOWLEDGMENTS This work was partially supported by the Ministry of Education, Japan, Grant No. 04558019, for which we are grateful. H.I. acknowledges Heian Jogakuin for the grant (Tokubetsu Kenkyuhi). REFERENCES (1) J. H. Bradbury, "The Structure and Chemistry of Keratin Fibers," in Advances in Protein Chemistry (Academic Press, New York and London, 1973), pp. 111-211. (2) D.J. Evans, J. D. Leeder, J. A. Rippon, and D. E. Rivett, Separation and analysis of the surface lipids of the wool fiber, Proc. 7th Int. Wool Textile Res. Conf, (Tokyo), vol. 1, 135-142 (1985). (3) H. Ito, and Y. Muraoka, Water transport along textile fibers as measured by an electrical capacitance technique, Textile Res. J., 63, 414-420 (1993). (4) J. Wilhelmy, Ober die Abhi/ngigkeit der Capillariti/ts-Constanten des Alkohols von Substanz und Gestalt des benetzten festen KiSrpers, Ann. Physik, 119, 177-217 (1863). (5) B. Miller and R. A. Young, Methodology for studying the wettability of filaments, Textile Res. J., 45, 359-365 (1975). (6) K. R. Makinson, "Practical Methods of Shrinkproofing", in Shrinkproofing of Wool (Marcel Dekker, New York, 1979), pp. 295-326. (7) H. Ito, H. Sakabe, T. Miyamoto, and H. Inagaki, Fibrillation of cortex of Merino wool fibers by freezing-thawing treatment, Textile Res. J., 54, 397-402 (1984). (8) L. S. Penn and B. Miller, Advancing, receding, and "equilibrium" contact angles, J. Colloid Interface Sci., 77, 574-576 (1980). (9) Y. K. Kamath, C. J. Dansizer, and H.-D. Weigman, Wetting behavior of human hair fibers, J. Appl. Polym. Sci., 22, 2295-2306. (10) Y.-L. Hsieh, M. Wu, and D. Andres, Wetting characteristics of poly (p-phenylene terephthalamide) single fibers and their adhesion to epoxy, J. Colloid Interface Sci., 144, 127-144 (1991). (11) Washburn, E. W., The dynamics of capillary flow, Phys. Rev., 17, 273-283 (1921).
j. Soc. Cosmet. Chem., 45, 193-202 (July/August 1994) Determination of imidurea in cosmetic products by capillary zone electrophoresis and miceliar electrokinetic chromatography GEORGE M. MICHALAKIS, Novo Nordisk Pharmaceutical Industries, Inc., 2231 Powhatan Road, Clayton, NC 27520, and EUGENE F. BARRY, Department of Chemistry, University of Massachusetts, Lowell, MA 01854. Received August 30, 1993. Synopsis Capillary electrophoresis has been employed for the determination of imidurea (imidazolidinyl urea) in cosmetic preparations. When dissolved in water, imidurea consists of 96.6 percent neutral species at a relative standard deviation (RSD) of 0.2 percent and the remaining 3.4 percent is associated with at least twenty anionic species. In addition, the micellar electrokinetic chromatography (MEKC) has been used for the determination of imidurea in the presence of paraben preservatives. The presence of micelles does not affect the imidurea elution profile due to the highly hydrophilic character of the imidurea species observed in this study. The preservatives in several commercial products have been identified and quantitated. INTRODUCTION In the formulation of cosmetic products, preservatives play an important role, since most cosmetics provide an ideal medium for bacterial growth. The parabens have been the most widely used class of preservatives. However, imidurea preservatives became avail- able to cosmetic chemists two decades ago, and their popularity has been steadily increasing. Imidurea is claimed to be nontoxic and nonirritating, with a broad spectrum of antimicrobial activity (1,2). Furthermore, due to the synergistic effect of imidurea with parabens, the two types of preservatives are typically used in combination. Despite the extensive use of imidurea, there are only three analytical procedures ap- pearing in the literature describing imidurea determinations (3-5). Ryder (3) utilized thin-layer chromatography (TLC) and illustrated separations requiring a development time of 50 minutes and an additional 20 minutes at 150øC for visualization. Two spots were observed for the imidurea standard (Rf of 0.27 and 0.35) over a concentration range of 0.1 to 0.6 percent (RSD -- l0 percent, n = 10), and an additional four bands were noted at higher loading experiments. Wilson presented a very extensive TLC procedure focusing on twenty-five preservatives with six indicating reagents where imidurea and propylparaben exhibited Rf values of 0 and 0.6, respectively (4), although detection 193
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)