220 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (48) (49) (50) (51) (52) (53) (54) (55) (56) (57) (58) (59) (60) B. Melnik, J. Hollman, U. Hoffman, M. S. Yuh, and G. Piewig, Lipid composition of outer stratum corneum and nails in atopic and control subjects, Arch. Dermatol. Res., 282, 549-551 (1990). G. Imokawa, A. Abe, K. Jin, Y. Higaki, M. Kawashima, and A. Hidano, Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin, J. Invest. Dermatol., 96, 523-526 (1991). A. Yamamoto, S. Serizawa, M. Ito, and Y. Sato, Stratum corneum lipid abnormalities in atopic dermatitis, Arch. Dermatol. Res., 282, 219-223 (1991). S. E. Friberg and I. Kayali, Water evaporation rates from a model of stratum corneum lipids, J. Pharm. Sci., 78, 639-643 (1989). P. W. Wertz and D. T. Downing, Epidermal ceramide hydrolase, J. Invest. Dermatol., 94, 590 (1990). D. L. Bissett and J. F. McBride, "Use of the Domestic Pig as an Animal Model of Human Dry Skin," in Models in Dermatology, H. Maibach and P. Lowe, Eds. (Karger, Basel, 1985), Vol 1, pp. 159-168. B. D. McKersie, J. H. Crowe, and L. M. Crowe, Free fatty acid effects on leakage, phase properties and fusion of fully hydrated model membranes, Biochim. Biophys. Acta, 982, 156-160 (1989). G. K. Menon, K. R. Feingold, M. Mao-Qiang, M. Schaude, and P. M. Elias, Structural basis for the barrier abnormality following inhibition of HMG CoA reductase in murine epidermis, J. Invest. Dermatol., 98, 209-219 (1992). H. Kido, N. Fukusen, and N. Katunuma, Inhibition of chymase activity by long chain fatty acids, Arch. Biochem. Biophys., 230, 610-614 (1984). R. White and M. Walker, Thermotropic and lyotropic behaviour of epidermal lipid fraction, Blochem. Soc. Trans., 18, 881-882 (1990). J. L. Leveque, G. L. Grove, J. Rigal, P. Corcuff, A.M. Kligman, and D. Saint Leger, Biophysical characterization of dry facial skin, J. Soc. Cosmet. Chem., 82, 171-177 (1987). I. Horij, Y. Nakayama, M. Obata, and H. Tagami, Stratum corneum hydration and amino acid content in xerotic skin, Br. J. Dermatol., 121, 282-284 (1989). A.M. Kligman, "The Biology of the Stratum Corneum," in The Epidermis, W. Montagna and W. C. Lobitz, Eds. (Academic Press, New York, 1964), pp. 387-433.
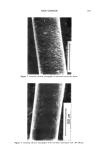
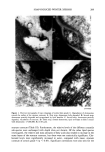
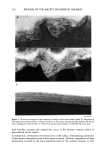
j. Soc. Cosmet. Chem., 45, 221-225 (July/August) An improved procedure for quantitative analysis of sebum production using Sebutape © A. PAGNONI, A. M. KLIGMAN, S. EL GAMMAL, C. POPP, and T. STOUDEMAYER, Foundation for Basic Cutaneous Research, Philadelphia, PA 19103 (A. P., C.P. ), Department of Dermatology, University of Pennsylvania, Philadelphia, PA 19104 (A.M.K. ), Department of Dermatology, Ruhr-University, Bochum 44791, Germany (S.e.G.), and S.K.I.N. Inc., Conshohocken, PA 19428 (T.S.). Received June 3, I994. Synopsis Sebutape © is an adhesive, white tape specifically designed for the collection of sebum. As sebum issues from the orifices, it is trapped in microcavities in the tape, yielding a pore pattern that can be quantified in regard to the number of spots, total area, and size distribution. We have become aware of substantial alterations in pore patterns when Sebutape © is stored for later analysis. These changes relate to enlargement of spots, decrease in their transparency, and increased translucency of the tape itself. We analyzed the evolution of these changes over time and in different storage conditions. We found that placing the Sebutape © on a plastic sheet and storing it in the freezer largely prevented these alterations. Room temperature storage is acceptable only if the samples are evaluated within 24 hours. Analysis immediately after removal yields the least artefacts. Recognizing the importance of changes associated with storage enhances the reliability and accuracy of the Sebutape © method. INTRODUCTION Sebutape © consists of a porous adhesive tape that has been designed to collect sebum on a sebaceous rich area, notably the face. As sebum issues from the follicular orifices, it is trapped within microcavities of the tape and becomes a transparent spot. The area occupied by spots per cm 2 is a measure of sebum production, while the number of spots reflects actively secreting follicles (1-3). Important technological improvements have occurred since the method was first de- scribed. Most recently, image analysis has been used to enable rapid quantitative anal- ysis (2). Usually, the Sebutape © is removed after one hour and placed upon a black card for storage and later analysis. We have become aware of serious changes in the pore patterns after varying periods of storage. In particular, we have noted several changes that can substantially distort the results. These are: a) enlargement of the spots, b) decreased 221
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)