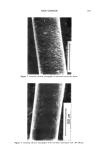
198 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS hours. After four hours, imidurea, initially white in color, turned yellow, and after twelve hours the raw material exhibited a yellow-orange color. At the end of the four- and twelve-hour heating periods, aqueous solutions of concentration of 5.0 and 6.0 mg/mL were prepared, respectively. The degraded imidurea substance did not dissolve completely in water, requiring filtration of the solutions. Parallel electropherograms of aqueous solutions containing fresh and thermally degraded imidurea are compared in Figure 3. After heating the raw material for four hours, the bands with migration times of about six and eight minutes are enhanced significantly, and new species with migration times greater than 12 minutes are detected (Figure 3B). 14. A 0 2 • 6 8 10 1'2 14 16 18 Minutes Figure 3. Parallel electropherograms of a freshly prepared imidurea solution (A), and aqueous solutions prepared after imidurea was subjected to 180øC for four hours (B) and twelve hours (C).
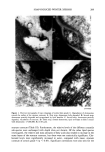
DETERMINATION OF IMIDUREA 199 u 40 2o 1 2 3 4 t, - o • , • • io i2 i4 i• Minutes Figure 4. A: MEKC electropherogram of the separation of (1) imidurea, 0.693 mg/mL (2) methylparaben, 0.135 mg/mL (3) ethylparaben, 0.044 mg/mL and (4) propylparaben, 0.053 mg/mL. Buffer: 10 mM phosphate at pH of 6.1 and 40 mM SDS. B: Almay, 32 mg/mL in buffer solution. C: Massengill, diluted 4:10 with buffer solution. An additional 60 percent of the original imidurea component degrades during this heating interval. After 12 hours at 180øC, the corresponding electropherogram (Figure 3C) illustrates distinct peaks at approximately ! 1 and !9 minutes while bands with elution times between six and nine minutes have decreased significantly. Approximately 66 percent of the imidurea component undergoes thermal degradation during the 12- hour heating period. QUANTITATION OF PRESERVATIVES IN COMMERCIAL PRODUCTS Separation of commonly encountered parabens from imidurea was achieved by MEKC
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)