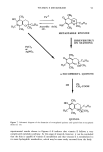
DEPOSITION OF GLYCOLIC ACID AND GLYCEROL 99 glycerol in distilled water containing trace amounts of 14C-glycolic acid or 3H-glycerol was heated to 70øC in a water bath. The pH of the 40 mg/ml aqueous glycolic acid solution was measured to be 1.9. The two syringes were connected via a three-way Teflon stopcock, and the aqueous phase was rapidly injected into the lipid phase. The contents were then injected back and forth between the two syringes while being cooled under cold tap water. This process was continued till the mixture was at room tem- perature. The resulting liposomal suspensions were then examined by inverted light microscopy to assure the quality and integrity of the liposomal preparations. The total lipid concentration in all liposomal preparations was 50 mg/ml. The concentrations of glycolic acid and glycerol were 40 mg/ml and 25 mg/ml, respectively. The formulations were stored at 4øC overnight before use in the experiments. 30% PG/water solution. Glycolic acid or glycerol solutions were prepared using a solvent mixture containing 30% (w/w) propylene glycol in water. The glycolic acid and glycerol concentrations were 40 mg/ml and 25 mg/ml, respectively. A trace amount of •4C- glycolic acid or 3H-glycerol was also included in the solutions. Oil-in-water emulsions. Appropriate amounts of mineral oil, Tween 60, and Arlacel 60 were accurately weighed in a scintillation vial. The vial was then capped and heated with stirring at 80øC in a water bath to dissolve the lipids in the oil. The aqueous phase consisting of distilled water or aqueous solution of glycolic acid or glycerol with trace amounts of •4C-glycolic acid or 3H-glycerol was preheated to 80øC. The aqueous phase was then added to the vial containing the lipid/oil mixture and vigorously stirred with cooling under cold water. The concentrations ofTween 60 and Arlacel 60 were 3% and 2% (w/w), respectively. The ratio of the aqueous to oil phases was 80:20 (w/w). The concentrations of glycolic acid and glycerol in the emulsions were 40 mg/ml and 25 mg/ml, respectively. The formulations were stored at 4øC overnight before use in the experiments. Water-in-oil emulsions. Appropriate amounts of mineral oil, paraffin soft, Arlacel 83, and Arlacel 60 were accurately weighed in a scintillation vial. The vial was then capped and heated with stirring at 60øC in a water bath to melt and dissolve the lipids in the oil phase. The mixed oil phase was then accurately weighed in a syringe and maintained at 60øC. The aqueous phase, consisting of distilled water or aqueous solutions of glycolic acid or glycerol containing trace amounts of •4C-glycolic acid or 3H-glycerol, respec- tively, was accurately weighed in another syringe at room temperature. The two syringes were connected via a three-way Teflon stopcock, and the aqueous phase was rapidly injected into the lipid phase. The contents were then injected vigorously back and forth between the two syringes while being cooled under cold tap water. The concentrations of Arlacel 83 and Arlacel 60 were 3% and 1% (w/w), respectively. The oil phase consisted of 40 wt% mineral oil and 5 wt% paraffin soft. The glycolic acid and glycerol concentrations were 40 mg/ml and 25 mg/ml, respectively. The water-to-oil ratio in the emulsions was 45:55 (w/w). The emulsions were stored at 4øC overnight before use in the experiments. In vivo deposition studies Male hairless mice (SKH-hr-1, 50-60 days old, Charles River Breeding Labs) were initially anesthetized with sodium pentobarbital (60 mg/Kg, J.p.). Twenty-five •1 of the test formulation were applied to a 4-cm 2 area of the dorsal skin surface of the mouse
100 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS and spread evenly over the entire surface. Additional periodic anesthetization was carried out with sodium pentobarbital (30 mg/Kg, i.p.) over the duration of the entire exper- iment. One hour following application, the formulation on the skin was removed by swabbing thrice carefully with Kimwipes ©. At 0, 2, 4, and 8 h following removal of formulation, the animals were sacrificed by a lethal dose injection of sodium pentobar- bital. Full-thickness dorsal skin was then carefully excised, and the liver and urinary bladder were harvested. The skin section was then mounted on a wooden board and stripped as follows: two pieces of adhesive tape (Scotch Magic © Tape, St. Paul, MN), each 1.9 cm wide and about 6 cm long, were placed alongside each other on the skin surface to strip the skin. The tapes were of sufficient size to cover the area of skin that was in contact with the test formulation. The skin was repeatedly stripped with fresh twin tapes, usually about 15 times, until it appeared shiny and glossy. The remaining skin and bladder, along with the surface swabs and strips, were then assayed for glycolic acid or glycerol content using a scintillation counter after addition of 15 ml of Ecolite(+) scintillation cocktail (ICN Biomedicals, Inc., Irvine, CA). The amount adhering to the stratum corneum surface is operationally defined as the amount found in the first two strippings. The amount of glycolic acid found in the stratum corneum is then defined as the amounts found in tape strippings 3 through 15. The amount of glycolic acid in the living skin strata is defined as that determined by analysis of the remainder of the full-thickness skin. Liver samples were analyzed following combustion in a Model 306 tissue oxidizer (Packard Instruments). All experiments were carried out under non-occluded conditions, and a minimum of three animals per formulation per time point were used. In vitro diffusion experiments Male hairless mice (SKH-hr-1, 50-60 days old, Charles River Breeding Labs) were sacrificed by lethal dose injection of sodium pentobarbital, and full-thickness dorsal skin was excised. Subcutaneous fat was carefully removed using a dull scalpel. Appropriate- sized pieces of skin were then mounted on Franz diffusion cells with a surface area of 1.77 cm 2 and a receiver capacity of 7 ml (Crown Glass, Somerville, NJ). The epidermal side of the skin was exposed to ambient conditions while the dermal side was bathed by a 0.05 M isotonic HEPES, pH 7.4, buffer. The receiver solution was stirred continu- ously using a small Teflon-covered magnet. Care was exercised to remove any air bubbles between the dermis side of the skin and the receiver solution. The temperature of the receiver solution was maintained at 37øC. Following mounting of the skin, 25 }xl of the test formulation was applied to the epidermal surface of the hairless mouse skin and carefully spread evenly to achieve complete surface coverage. A minimum of three cells, using skin from at least three different animals, was used for each system tested. At 16 h, the diffusion setup was dismantled. Upon dismantling, the donor cap was rinsed in 10 ml of distilled water followed by a 20-ml methanol rinse. The methanol rinse was allowed to dry in a hood, at which time scintillation cocktail was added. The skin piece was then mounted on a board, and the epidermal side of the skin was swabbed carefully three times with a dry Kimwipe © to remove any undried formulation. The skin was then stripped repeatedly as follows: A piece of adhesive tape (Scotch Magic Tape, 810, 3M Commercial Office Supply Division, St. Paul, MN), 1.9 cm wide and about 6 cm long, was used to strip the skin. The tape was of sufficient size to cover the area of skin that was in contact with the test formulation. At least nine strippings were carried out, and
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)