168 JOURNAL OF COSMETIC SCIENCE The number of droplets in the diluted emulsion was counted in ten size ranges and a volume-number mean diameter was calculated according to the following equation (10): where n i is the number of droplets of diameter di. CREAMING MEASUREMENTS Creaming was measured by a visual technique. An amount of 10 ml of each formed emulsion was stored in a closed volumetric cylinder of 10 ml at 25 o + 1 øC. The volume of the formed cream was measured one month after the preparation. RESULTS AND DISCUSSION EMULSION AREAS Ternary phase diagrams, presented in Figure 1, show the influence of the glycerol:water mass ratio on the area of existence of stable oil-in-water emulsions. Stable oil-in-water emulsions are formed with any mixture of glycerol and water. Stable oil-in-water emul- sions with an isopropyl myristate concentration higher than 75 % could not be prepared under the experimental conditions of this study. Also, systems with a polysorbate 80 concentration higher than 20% could not be prepared. Phase studies on these systems, for the polysorbate 80 concentration higher than 20%, showed the existence of oil-in- water microemulsion and microemulsion gel regions (11). VISCOSITY The rheological behavior of emulsions was determined 24 hours after the preparation. The results with the HAAKE viscometer showed that the viscosity of each emulsion remains stable for different values of shear rate, namely that the examined emulsions were NewtonJan systems. The viscosity of emulsions with a value under 20 mPa s was confirmed with Ubbelohde suspended-level viscometers. The results are shown in the last columns of Tables I and II. An increase in viscosity with an increase in glycerol concentration in the external phase of the emulsions (Table I) is expected, because glycerol, as a very viscous liquid, increases the viscosity of an emulsion exponentially when added in the external phase. There are more viscous agents than glycerol in order to increase the viscosity of the external phase of an emulsion, such as methylcellulose, but glycerol is more convenient when the form is destined for topical pharmaceutical, as well as cosmetic, use (12). The decrease in the viscosity of the emulsions, with the increase in the polysorbate 80 concentration, at surfactant concentrations up to 1% by weight (Table II), is attributed to the decrease in droplet size with the increase in the surfactant concentration. On the other hand, at concentrations higher than 1%, the polysorbate 80 does not remain just
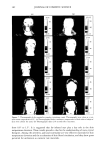
EMULSION STABILITY 169 o o o G G o S loo o S loo o S loo (a) (b) (c) o loo o loo /•oo looq•,._• • •l 0 loo 0 1•• i•pploo 0 S loo 0 S loo 0 S (d) (e) o o o lOO loo loo , o o s loo o s loo o s loo (g) (h) (i) Figure 1. Ternary phase diagrams showing areas of existence of oil-in-water emulsions with isopropyl myristate (O), polysorbate 80 (S), glycerol (G), and water (W) at 40øC for glycerol:water mass ratios of: (a) 1:9, (b) 2:8, (c) 3:7, (d) 4:6, (e) 5:5, (f) 6:4, (g) 7:3, (h) 8:2, and (i) 9:1. in the interface, but it also dissolves in the external phase, having as a result an increase in the viscosity of the emulsions, since polysorbate 80 is more viscous than water. Therefore, an emulsifier concentration under 1% by weight should not be used since it is insufficient for stable emulsions. This result also shows that a rheological study of an emulsion system with a varying emulsifier concentration is recommended in order to detect in practice the least possible emulsifier concentration, which will allow the formation of a stable emulsion system.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)