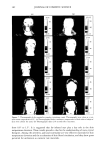
172 JOURNAL OF COSMETIC SCIENCE 3.5 • 2.5 e 2.0 ß ß g' • 1.13 O 0.5 0.0 ' 0 10 20 30 40 50 60 70 Glycerol % w/w Figure 4. Creaming of emulsion as a function of the glycerol concentration. o o 5 lO 15 20 25 Polysorbate 80 % w/w Figure 5. Creaming of emulsion as a function of the polysorbate 80 concentration. 80 is in excess and dissolved in the external phase as well. For the first reason, the decrease in the creaming with the increase in the polysorbate 80 concentration is more dramatic than it is for the second reason, where the decrease in the creaming with the increase in the polysorbate 80 concentration is linear. CONCLUSIONS From the results above, it is obvious that with a polysorbate 80 concentration of more than 1% by weight, the viscosity increases due to the dissolution of emulsifier in the
EMULSION STABILITY 17 3 external phase. A special reduction in droplet size is not observed when the emulsifier concentration becomes more than 5% by weight. In addition, an emulsifier concentra- tion of about 5 % by weight is out of the zone of the dramatic creaming reduction. Based on all these, it can be proposed that polysorbate 80 be used at a concentration of 5 % by weight. ACKNOWLEDGMENTS The authors wish to express their thanks to the state Scholarship Foundation of Greece for the scholarship provided to C. A. Ayannides. REFERENCES (1) F. Liu and D. Liu, Long circulation emulsions (oil-in-water) as carriers for lipophilic drugs, Pharm. Res., 12, 1060-1064 (1995). (2) T. Takino, K. Konishi, Y. Takakura, and M. Hashida, Long circulating emulsion carrier systems for highly lipophilic drugs, Biol. Pharm. 17, 121-125 (1994). (3) B. Idson, "Pharmaceutical Emulsions," in PharmaceuticalDosage Forms: Disperse Systems, H. A. Liberman, M. M. Rieger, and G. S. Banker, Eds. (Marcel Dekker, New York, Basel, 1988), Vol. 1, pp. 236-240. (4) A. T. Florence and D. Artwood, "Emulsions," in Physicochemical Principles of Pharmacy, 3rd ed., A. T. Florence and D. Artwood, Eds. (Macmillan, London, 1998), pp. 264-266. (5) P. Prinderre, Ph. Piccerelle, E. Cauture, G. Kalantzis, J.P. Reynier, and J. Joachim, Formulation and evaluation of o/w emulsions using experimental design, Int. J. Pharm., 163, 73-79 (1998). (6) G. Ktistis and N. Iconomou-Petrovic, The influence of the concentration and HLB value of the emulsifying agents on the stability of emulsions, Pharm. Ddtion, 7, 1-9 (1981). (7) R. P. Gullapalli and B. B. Sheth, Effect of methylcellulose on the stability of oil-in-water emulsions, Int. •. Pharm., 140, 97-109 (1996). (8) E. Shotton and S.S. Davis, The use of the Coulter counter for the particle size analysis of some emulsion systems, J. Pharm. PharmacoL, 20, 430•438 (1968). (9) F. Kiekens, A. Vermeire, N. Samyn, J. Demeester, and J. P. Remon, Optimization of electrical conductance measurements for the quantification and prediction of phase separation in o/w emulsions containing hydroxypropylmethylcelluloses as emulsifying agents, Int. •. Pharm., 146, 239-245 (1997). (10) A. Martin, P. Bustamante, and A. H. C. Chun, "Micromeritics," in Physical Pharmacy, 4th ed., A. Martin, P. Bustamante, and A. H. C. Chun, Eds. (Lea and Febiger, Philadelphia, London, 1993), pp. 425•426. (11) C.A. Ayannides and G. Ktistis, A theological study on microemulsion gels of isopropyl myristate, polysorbate 80, glycerol, and water, J. Cosmet. Sci., 50, 1-7 (1999). (12) C. Valenta, E. Nowack, and A. Bernkop-Schnurch, Deoxycholate-hydrogels: Novel drug carrier sys- tems for topical use, Int. •. Pharm., 185, 103-111 (1999). (13) T. F. Tadros and B. Vincent, "Emulsion Stability," in Encyclopedia of Emulsion Technology, P. Bether, Eds. (Mercel Dekker, New York, Basel, 1983), p. 147.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)